Abstract
Donor–π spacer–acceptor (D–π–A) dyes are among the most attractive structures for the design of organic dye-sensitized solar cells (DSSCs). Typically, the key intermediates for these sensitizers are D–π compounds containing an aldehyde group to which an anchor acceptor group is attached via the Knoevenagel reaction. In this communication, 5-(9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazol-6-yl)thiophene-2-carbaldehyde was prepared via the Suzuki cross-coupling reaction. The structure of the newly synthesized compound was established by means of high-resolution mass spectrometry, 1H NMR, 13C NMR, IR, and UV–Vis spectroscopy. The title compound would be used in the synthesis of sensitizers for DSSCs.
1. Introduction
In recent years, solar-to-electricity converters such as organic solar cells have gained significant interest, not only theoretically but also in practice [,]. Using organic materials instead of traditional inorganic ones offers many advantages, such as ease of device manufacture, low cost, universal molecular design, and ease of control over the physical properties of materials [,]. Dye-sensitized solar cells (DSSCs) based on organic dyes are one of the most promising devices for the transformation of solar energy [,]. However, industrial applications of organic solar cells are hampered by issues related to cost, synthesis scalability, and the stability of organic dyes [,,]. Organic sensitizers for DSSCs, as a rule, have a D–π–A-type donor–acceptor structure, in which the acceptor also acts as an anchor during adsorption on the surface of nanocrystalline TiO2 [,]. In this case, the donor (D) and acceptor (A) parts are connected to each other through a π bridge, designed to expand the conjugation chain and increase the distance traveled by the electron during excitation []. Typically, in the synthesis of D–π–A dyes, the last step is the well-known Knoevenagel reaction of D–π–aldehyde with cyanoacrylic acid [,], and Suzuki cross-couplings are most often employed for the preparation of a D–π–aldehyde-type molecule [,]. Therefore, aldehyde synthesis is a key step in the preparation of the sensitizer. Herein, we report the synthesis of 5-(9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazol-6-yl)thiophene-2-carbaldehyde 2 as a precursor for the preparation of DSSCs components.
2. Results and Discussion
To synthesize the target 5-(9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazol-6-yl)thiophene-2-carbaldehyde 2, we studied the direct C–H activation reactions between 6-bromo-9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazole 1 (R = Br) [] and thiophene-2-carbaldehyde (X = H) under the conditions previously described in the literature for similar transformations (Scheme 1) []. However, under the conditions studied, the reaction did not occur, and the starting compounds were recovered in almost quantitative yield (Table 1, entries 1–2). The Suzuki reaction of 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazole 1 (R = Bpin2) [] with 5-bromothiophene-2-carbaldehyde (X = Br) resulted in compound 2 in moderate yield (Table 1, entry 3).
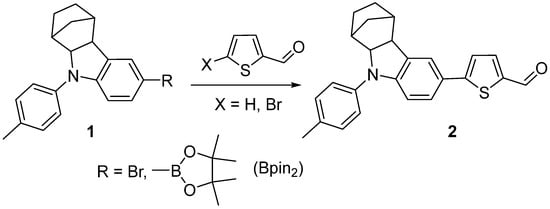
Scheme 1.
Synthesis of 5-(9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazol-6-yl)thiophene-2-carbaldehyde 2.

Table 1.
Reaction of 9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazoles 1 with thiophene-2-carbaldehydes.
The structure of 5-(9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazol-6-yl)thiophene-2-carbaldehyde 2 was confirmed by means high-resolution mass spectrometry, 1H NMR, 13C NMR, and IR spectroscopy. For compound 2, the UV–Vis absorption spectrum was recorded in a solution in DCM, which showed the presence of two absorption maxima. In this case, the far-wave absorption maximum, responsible for the charge transfer from the donor to the acceptor (intramolecular charge transfer, CT band) [], had a value of 432 nm. The obtained results suggest that further functionalization of compound 2 at the aldehyde group may result in a bathochromic shift of the CT band, which may lead to D–π–A-type dyes with a wide spectral range overlap.
3. Materials and Methods
6-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazole 1 (R = Bpin2) was prepared according to the published method []. The solvents and reagents were purchased from commercial sources and used as received. 1H and 13C NMR spectra were taken with a Bruker AM-300 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA), at frequencies of 300 and 75 MHz, in CDCl3 solutions, with TMS as the standard. J values are presented in Hz. The MS spectrum (EI, 70 eV) was obtained with a Finnigan MAT INCOS 50 instrument (Hazlet, NJ, USA). The IR spectrum was measured with a Bruker “Alpha-T” instrument in the KBr pellet. High-resolution MS spectrum was measured on a Bruker micrOTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) using electrospray ionization (ESI). The UV–Vis absorption spectrum was recorded using an OKB Spektr SF-2000 UV/Vis/NIR spectrophotometer (Saint Petersburg, Russia), controlled with SF-2000 software in standard 10 mm photometric quartz cells using HPLC-grade DCM in a concentration of 2 × 10−5 M.
The spectra for 5-(9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazol-6-yl)thiophene-2-carbaldehyde 2 are provided in Supplementary Materials.
In a 50 mL round-bottom flask, 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazole 1 (317 mg, 0.79 mmol) and 5-bromothiophene-2-carbaldehyde (189 mg, 0.99 mmol) were dissolved in THF (20 mL), and a solution of K2CO3 (109 mg, 0.79 mmol) in water (5 mL) was added. The mixture was degassed for 20 min with a stream of argon, and Pd(PPh3)4 (45 mg, 0.040 mmol) was added. After refluxing for 8 h, the reaction mixture was diluted using EtOAc (25 mL) and washed with water (3 × 30 mL). The organic layer was dried using Na2SO4 and then evaporated. The residue was purified via column chromatography on silica gel (Silica gel Merck 60, with eluent a mixture of petroleum ether (40–60 °C) and EtOAc, 100:1, v/v). Yield 191 mg (63%, 0.496 mmol), orange solid, mp 205–206 °C. 1H NMR (300 MHz, CDCl3, δ, ppm): 9.82 (s, 1H), 7.67 (d, J = 3.9, 1H), 7.42–7.13 (m, 7H), 6.76 (d, J = 8.3, 1H), 4.31 (d, J = 8.1, 1H), 3.32 (d, J = 8.3, 1H), 2.52–2.38 (m, 2H), 2.35 (s, 3H), 1.59–1.35 (m, 4H), 1.31–1.16 (m, 2H). 13C NMR (75 MHz, CDCl3, δ, ppm): 182.4, 156.3, 151.2, 140.0, 139.8, 138.1, 134.4, 132.9, 130.0, 126.6, 123.1, 122.9, 121.5, 121.3, 107.1, 71.9, 50.0, 43.7, 40.9, 32.4, 28.6, 25.3, 20.9. HRMS-ESI (m/z): [M+H]+ calcd for (C25H23NOS) 386.1528, found 386.1535. UV-Vis (CH2Cl2, λmax, nm/logε): 297/4.05, 432/4.40. IR, ν, cm−1: 2950, 2916, 2865, 1729, 1646, 1600, 1513, 1450, 1434, 1372, 1272, 1235, 1059, 800. Rf = 0.33 (petroleum ether/EtOAc—10:1).
Supplementary Materials
The following are available online: copies of 1H, 13C NMR, IR, UV, and HR mass spectra for compound 2.
Author Contributions
Conceptualization, E.A.K.; methodology, O.A.R.; software, E.A.K.; validation, O.A.R.; formal analysis, investigation, N.S.G.; resources, O.A.R.; data curation, N.S.G.; writing—original draft preparation, E.A.K.; writing—review and editing, E.A.K.; visualization, N.S.G.; supervision, O.A.R.; project administration, O.A.R.; funding acquisition, O.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, Grant Number 22-73-00102.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Prajapat, K.; Dhonde, M.; Sahu, K.; Bhojane, P.; Murty, V.; Shirage, P.M. The Evolution of Organic Materials for Efficient Dye-Sensitized Solar Cells. J. Photochem. Photobiol. C Photochem. Rev. 2023, 55, 100586. [Google Scholar] [CrossRef]
- Riede, M.; Spoltore, D.; Leo, K. Organic Solar Cells—The Path to Commercial Success. Adv. Energy Mater. 2021, 11, 2002653. [Google Scholar] [CrossRef]
- Sen, A.; Putra, M.H.; Biswas, A.K.; Behera, A.K.; Groβ, A. Insight on the Choice of Sensitizers/Dyes for Dye Sensitized Solar Cells: A Review. Dye. Pigment. 2023, 213, 111087. [Google Scholar] [CrossRef]
- Yahya, M.; Bouziani, A.; Ocak, C.; Seferoğlu, Z.; Sillanpää, M. Organic/Metal-Organic Photosensitizers for Dye-Sensitized Solar Cells (DSSC): Recent Developments, New Trends, and Future Perceptions. Dye. Pigment. 2021, 192, 109227. [Google Scholar] [CrossRef]
- Lee, C.-P.; Li, C.-T.; Ho, K.-C. Use of Organic Materials in Dye-Sensitized Solar Cells. Mater. Today 2017, 20, 267–283. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Duan, T.; Feng, W.; Li, Y.; Li, Z.; Zhang, Z.; Liang, H.; Chen, H.; Zhong, C.; Jeong, S.; Yang, C.; et al. Electronic Configuration Tuning of Centrally Extended Non-Fullerene Acceptors Enabling Organic Solar Cells with Efficiency Approaching 19%. Angew. Chemie Int. Ed. 2023, 62, e202308832. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Bi, X.; Chen, H.; He, T.; Lin, Y.; Zhang, Y.; Ma, K.; Feng, W.; Ma, Z.; Long, G.; et al. A Rare Case of Brominated Small Molecule Acceptors for High-Efficiency Organic Solar Cells. Nat. Commun. 2023, 14, 4707. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Yang, J.; Xia, J.; Shu, H.; Yao, X.; Luo, J.; Jia, C. Simple Molecular Structure but High Efficiency: Achieving over 9% Efficiency in Dye-Sensitized Solar Cells Using Simple Triphenylamine Sensitizer. J. Power Sources 2021, 506, 230214. [Google Scholar] [CrossRef]
- Singh, M.; Nadendla, S.; Kanaparthi, R.K. Unravelling the Effect of Donor-π-Acceptor Architecture in Designing 1,3-Indanedione Based Sensitizers for DSSC Applications. J. Photochem. Photobiol. A Chem. 2023, 435, 114328. [Google Scholar] [CrossRef]
- Sun, K.; Wang, L.; Mao, L.; Zhang, Y.; Liu, F.; Zhang, J. Influence of π Spacer of Donor-Acceptor-π-Acceptor Sensitizers on Photovoltaic Properties in Dye-Sensitized Solar Cells. Org. Electron. 2020, 76, 105429. [Google Scholar] [CrossRef]
- Marsya, M.A.; Hayati, D.; Han, S.; Long, D.X.; Choi, K.; Hong, J. UV-Harvesting Dyes Featuring a Fluorene Donor for Visibly Transparent and Colorless Dye-Sensitized Solar Cells. Dye. Pigment. 2022, 200, 110131. [Google Scholar] [CrossRef]
- Reddy-Marri, A.; Marchini, E.; Cabanes, V.D.; Argazzi, R.; Pastore, M.; Caramori, S.; Gros, P.C. Panchromatic Light Harvesting and Record Power Conversion Efficiency for Carboxylic/Cyanoacrylic Fe(ii) NHC Co-Sensitized FeSSCs. Chem. Sci. 2023, 14, 4288–4301. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, M.S.; Ustimenko, O.O.; Knyazeva, E.A.; Rakitin, O.A. 4,4-Bis(2-Ethylhexyl)-6-(9-(2-Ethylhexyl)-2,3,4,4a,9,9a-Hexahydro-1H-Carbazol-6-Yl)-4H-Cyclopenta[2,1-b:3,4-B′]Dithiophene-2-Carbaldehyde. Molbank 2022, 2022, M1486. [Google Scholar] [CrossRef]
- Gudim, N.S.; Mikhailov, M.S.; Knyazeva, E.A.; Almenningen, D.M.; Mikhalchenko, L.V.; Economopoulos, S.P.; Rakitin, O.A. Monitoring the Dependence of the Photovoltaic Properties of Dye-Sensitized Solar Cells from the Structure of D–A–π–A-Type Sensitizers with a 9-(p-Tolyl)-2,3,4,4a,9,9a-Hexahydro-1H-1,4-Methanocarbazole Donor Building Block. Mol. Syst. Des. Eng. 2022, 7, 755–766. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Kudryashev, T.A.; Alekhina, D.A.; Rakitin, O.A. Palladium-Catalyzed Direct (Het)Arylation Reactions of Benzo[1,2-d:4,5-D′]Bis([1,2,3]Thiadiazole and 4,8-Dibromobenzo[1,2-d:4,5-D′]Bis([1,2,3]Thiadiazole). Molecules 2023, 28, 3977. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chu, Y.; Xu, M.; Zhang, S.; Ye, H.; Hu, Y.; Hua, J. Effect of π-Bridge Groups Based on Indeno[1,2- b ]Thiophene D–A–π–A Sensitizers on the Performance of Dye-Sensitized Solar Cells and Photocatalytic Hydrogen Evolution. J. Mater. Chem. C 2020, 8, 14864–14872. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).