1-(3,4-Dimethoxyphenyl)-3-(4-methoxyphenyl)-3-(1H-1,2,4-triazol-1-yl)propan-1-one
Abstract
1. Introduction
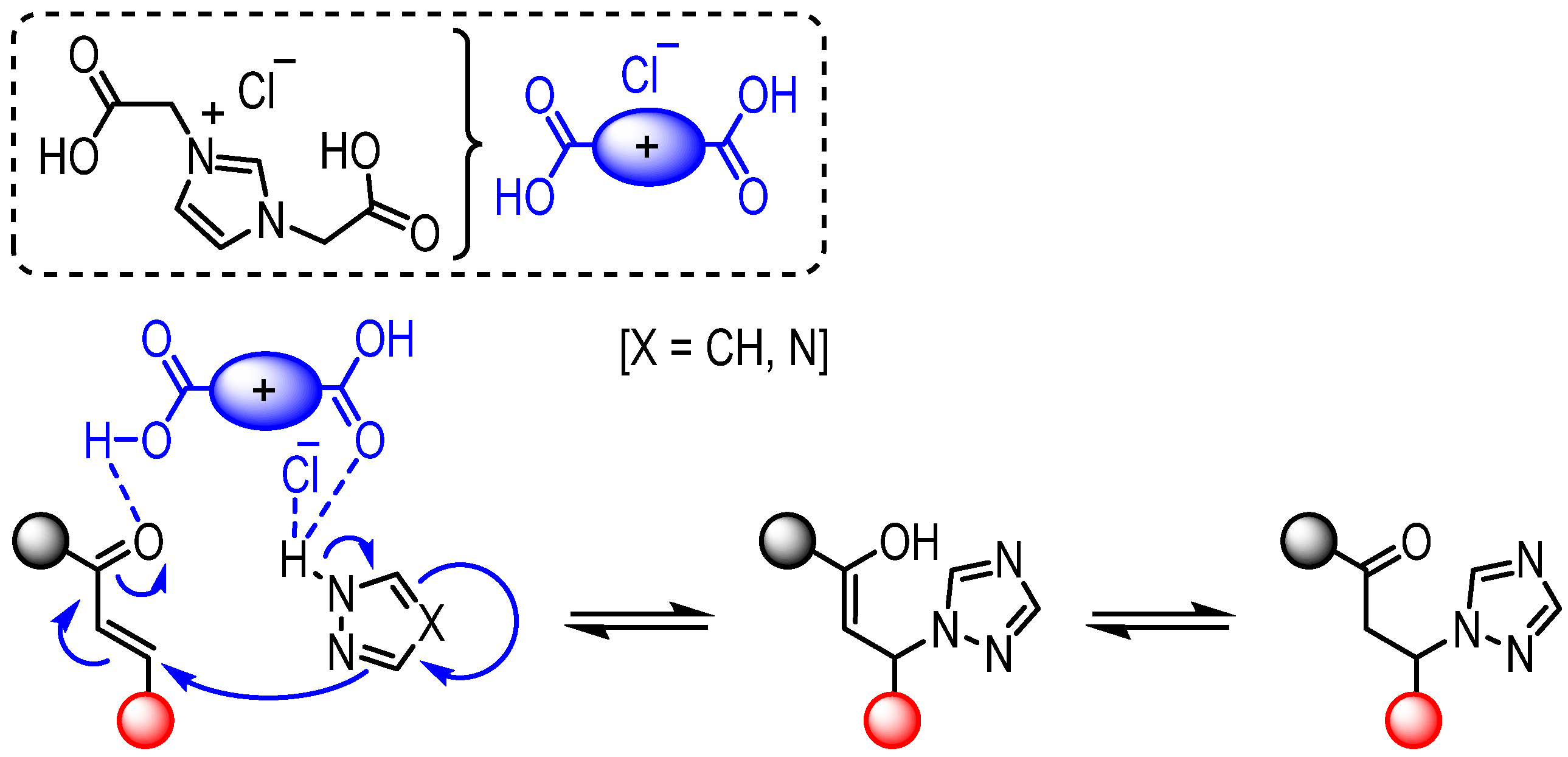
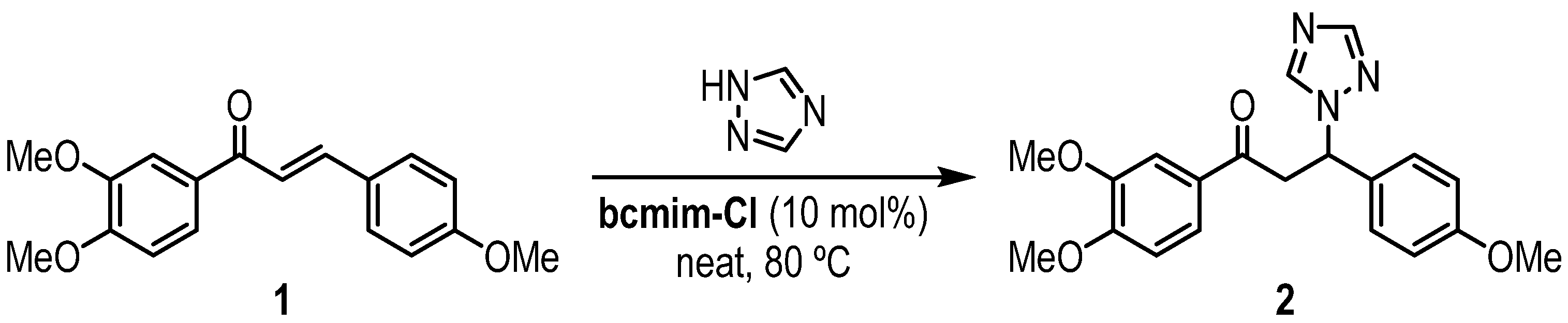
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Arora, P.; Arora, V.; Lamba, H.S.; Wadhwa, D. Importance of heterocyclic chemistry: A review. Int. J. Pharm. Sci. Res. 2012, 3, 2947–2954. [Google Scholar]
- Heravi, M.M.; Zardsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; He, S.-C.; Peng, Y.-J.; Zhang, H.-J.; Gopala, L.; Tangadanchu, V.K.R.; Gan, L.-L.; Zhou, C.-H. Design, synthesis and antimicrobial evaluation of novel benzimidazole-incorporated sulfonamide analogues. Eur. J. Med. Chem. 2017, 15, 136165–136183. [Google Scholar] [CrossRef]
- Min, Y.K.; Asami, T.; Fujioka, S.; Murofushi, N.; Yamaguchi, I.; Yoshida, S. New lead compounds for brassinosteroid biosynthesis inhibitors. Bioorg. Med. Chem. Lett. 1999, 9, 425–430. [Google Scholar] [CrossRef]
- Bhanuchandra, M.; Kuram, M.R.; Sahoo, A.K. A convenient approach to β-heteroarylated (C-N bond) ketones from Cs2CO3 promoted reaction between propargyl alcohols and nitrogen-heterocycles. Org. Biomol. Chem. 2012, 10, 3538–3555. [Google Scholar] [CrossRef]
- Ehrhardt, H.; Mildenberger, H.; Sachse, B.; Hartz, P. Fungicide and Bactericide 3-aryl-3-oxo-propyl-1,2,4-Triazoles Derivatives. DE-Patent Number DE3020500A1, 21 January 1982. [Google Scholar]
- Shephard, M.C.; Sugavanam, B.; Worthington, P.A.; Collins, D.J.; Griffin, D. Herbicidal Formulations. U.S. Patent Number US4113465, 12 September 1978. [Google Scholar]
- Marrapu, V.K.; Mittal, M.; Shivahare, R.; Gupta, S.; Bhandari, K. Synthesis and evaluation of new furanyl and thiophenyl azoles as antileishmanial agents. Eur. J. Med. Chem. 2011, 46, 1694–1700. [Google Scholar] [CrossRef]
- Kumar, L.; Sarswat, A.; Lal, N.; Jain, A.; Kumar, S.; Kumar, S.T.V.S.K.; Maikhuir, J.P.; Pandey, A.K.; Shukla, P.K.; Gupta, G. Design and synthesis of 3-(azol-1-yl)phenylpropanes as microbicidal spermicides for prophylactic contraception. Bioorg. Med. Chem. Lett. 2011, 21, 176–181. [Google Scholar] [CrossRef]
- Roman, G.; Comanita, E.; Comanita, B. Synthesis and reactivity of Mannich bases. Part 15: Synthesis of 3-(2-(1-pyrazolyl)ethyl)-1,2-benzisoxazoles. Tetrahedron 2002, 58, 1617–1622. [Google Scholar] [CrossRef]
- Srivastava, N.; Banik, B.K. Bismuth nitrate-catalyzed versatile Michael reactions. J. Org. Chem. 2003, 68, 2109–2114. [Google Scholar] [CrossRef]
- Lv, J.; Wu, H.; Wang, Y. Organocatalytic enantioselective aza-Michael additions of N-heterocycles to α,β-unsaturated enones. Eur. J. Org. Chem. 2010, 2010, 2073–2083. [Google Scholar] [CrossRef]
- Bayindir, S.; Erdogan, E.; Kilic, H.; Saracoglu, N. An efficient synthesis of new aza-substituted indoles via Michael-type addition. Synlett 2010, 10, 1455–1458. [Google Scholar] [CrossRef]
- Rulev, A.Y. Aza-Michael reaction: A decade later—Is the research over? Eur. J. Org. Chem. 2023, 26, e202300451. [Google Scholar] [CrossRef]
- Ying, A.; Zhang, Q.; Li, H.; Shen, G.; Gong, W.; He, M. An environmentally bening protocol: Catalyst-free Michael addition of aromatic amines to α,β-unsaturated ketones in glycerol. Res. Chem. Intermed. 2013, 39, 517–525. [Google Scholar] [CrossRef]
- Iida, H.; Tang, Z.; Yashiman, E. Synthesis and bifunctional asymmetric organocatalysis of helical poly(phenylacetylene)s bearing chinchona alkaloid pendants via a sulfonamide linkage. J. Polymer Sci. A Polymer Chem. 2013, 51, 2869–2879. [Google Scholar] [CrossRef]
- Selvi, T.; Velmathi, S. Indium(III) triflate-catalyzed reactions of aza-Michael adducts of chalcones with aromatic amines: Retro-Michael addition versus quinoline formation. J. Org. Chem. 2018, 83, 4087–4091. [Google Scholar] [CrossRef]
- Albert-Soriano, M.; Pastor, I.M. Anion-dependent imidazolium-based catalysts for allylation of aniline with tunable regioselectivity. Adv. Synth. Catal. 2020, 362, 2494–2502. [Google Scholar] [CrossRef]
- Martos, M.; Pérez-Almarcha, Y.; Pastor, I.M. DES-type interactions to promote solvent-free and metal-free reactions between nitrogen-containing heterocycles and allylic alcohols. Eur. J. Org. Chem. 2022, 2022, e202201221. [Google Scholar] [CrossRef]
- Nacher-Luis, A. Estudio Sobre Sales de Imidazolio Para Aplicaciones en Síntesis Orgánica; Degree Final Project; University of Alicante: Alicante, Spain, 2023; Available online: http://hdl.handle.net/10045/135324 (accessed on 28 February 2024).
- Ballini, R.; Bosica, G.; Maggi, R.; Ricciutelli, M.; Righi, P.; Sartori, G.; Sartorio, R. Clay-catalysed solventless synthesis of trans-chalcones. Green Chem. 2001, 3, 178–180. [Google Scholar] [CrossRef]
- Palleros, D.R. Solvent-free synthesis of chalcones. J. Chem. Educ. 2004, 81, 1345–1347. [Google Scholar] [CrossRef]
- Andraos, J. Reaction Green Metrics—Problems, Exercises, and Solution; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2019. [Google Scholar]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep eutectic solvents: The organic reaction medium of the century. Eur. J. Org. Chem. 2016, 2016, 612–632. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Stephens, N.M.; Smith, E.A. Structure of deep eutectic solvents (DESs): What we know, what we want to know, and why we need to know it. Langmuir 2022, 38, 14017–14024. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.; Ahmad, I.; Kahlon, A.K.; Hasanain, M.; Sharma, S.; Srivastava, K.K.; Sarkar, J.; Shankar, K.; Sharma, A.; Gupta, A. Syntheses of 2-methoxyestradiol and eugenol template based diarylpropenes as non-steroidal anticancer agents. RSC Adv. 2014, 4, 35171–35185. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nacher-Luis, A.; Pastor, I.M. 1-(3,4-Dimethoxyphenyl)-3-(4-methoxyphenyl)-3-(1H-1,2,4-triazol-1-yl)propan-1-one. Molbank 2024, 2024, M1791. https://doi.org/10.3390/M1791
Nacher-Luis A, Pastor IM. 1-(3,4-Dimethoxyphenyl)-3-(4-methoxyphenyl)-3-(1H-1,2,4-triazol-1-yl)propan-1-one. Molbank. 2024; 2024(1):M1791. https://doi.org/10.3390/M1791
Chicago/Turabian StyleNacher-Luis, Anna, and Isidro M. Pastor. 2024. "1-(3,4-Dimethoxyphenyl)-3-(4-methoxyphenyl)-3-(1H-1,2,4-triazol-1-yl)propan-1-one" Molbank 2024, no. 1: M1791. https://doi.org/10.3390/M1791
APA StyleNacher-Luis, A., & Pastor, I. M. (2024). 1-(3,4-Dimethoxyphenyl)-3-(4-methoxyphenyl)-3-(1H-1,2,4-triazol-1-yl)propan-1-one. Molbank, 2024(1), M1791. https://doi.org/10.3390/M1791






