Synthesis of (Z)-3-Allyl-5-(4-nitrobenzylidene)-2-sulfanylidene-1,3-thiazolidin-4-one and Determination of Its Crystal Structure
Abstract
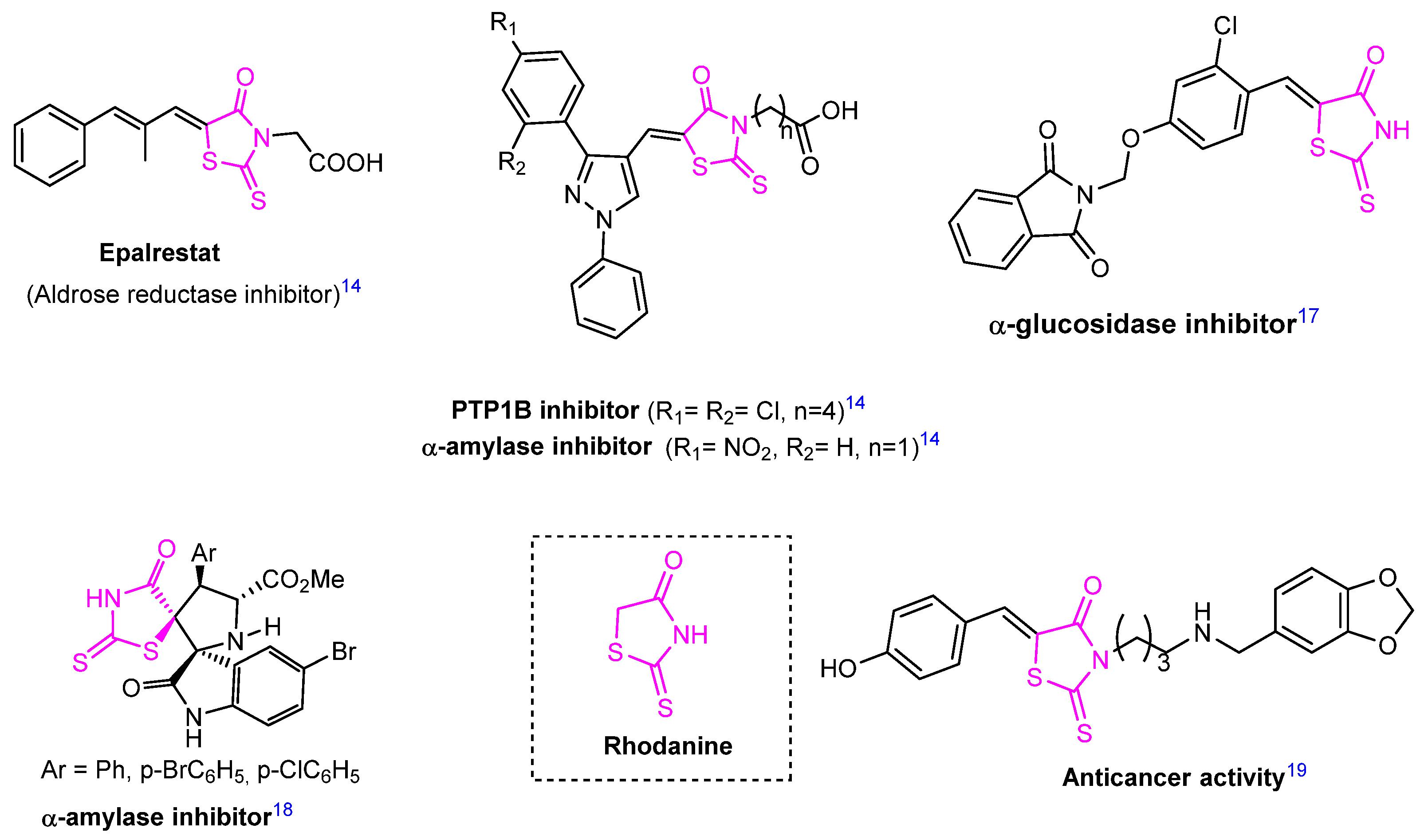
1. Introduction
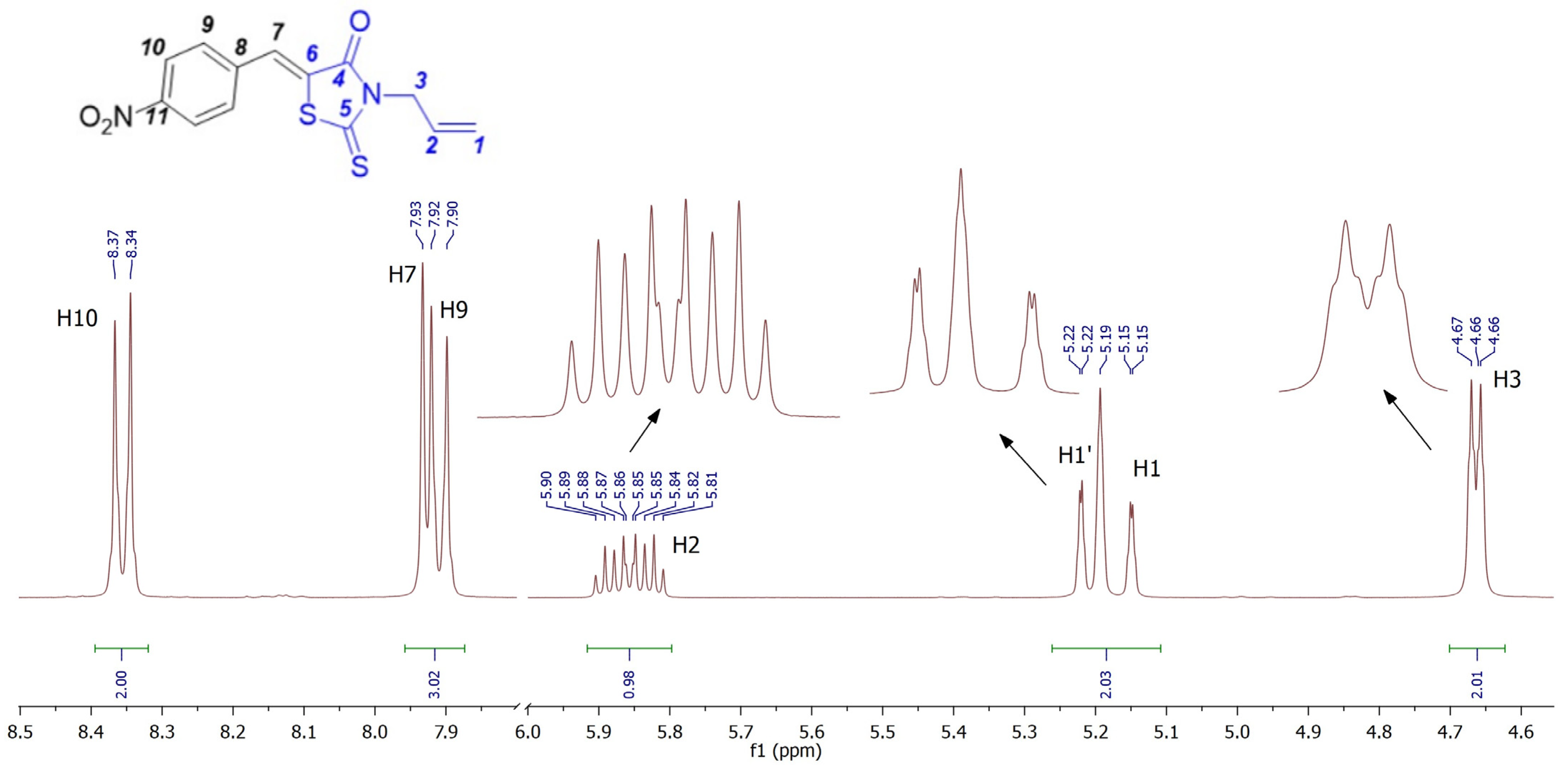
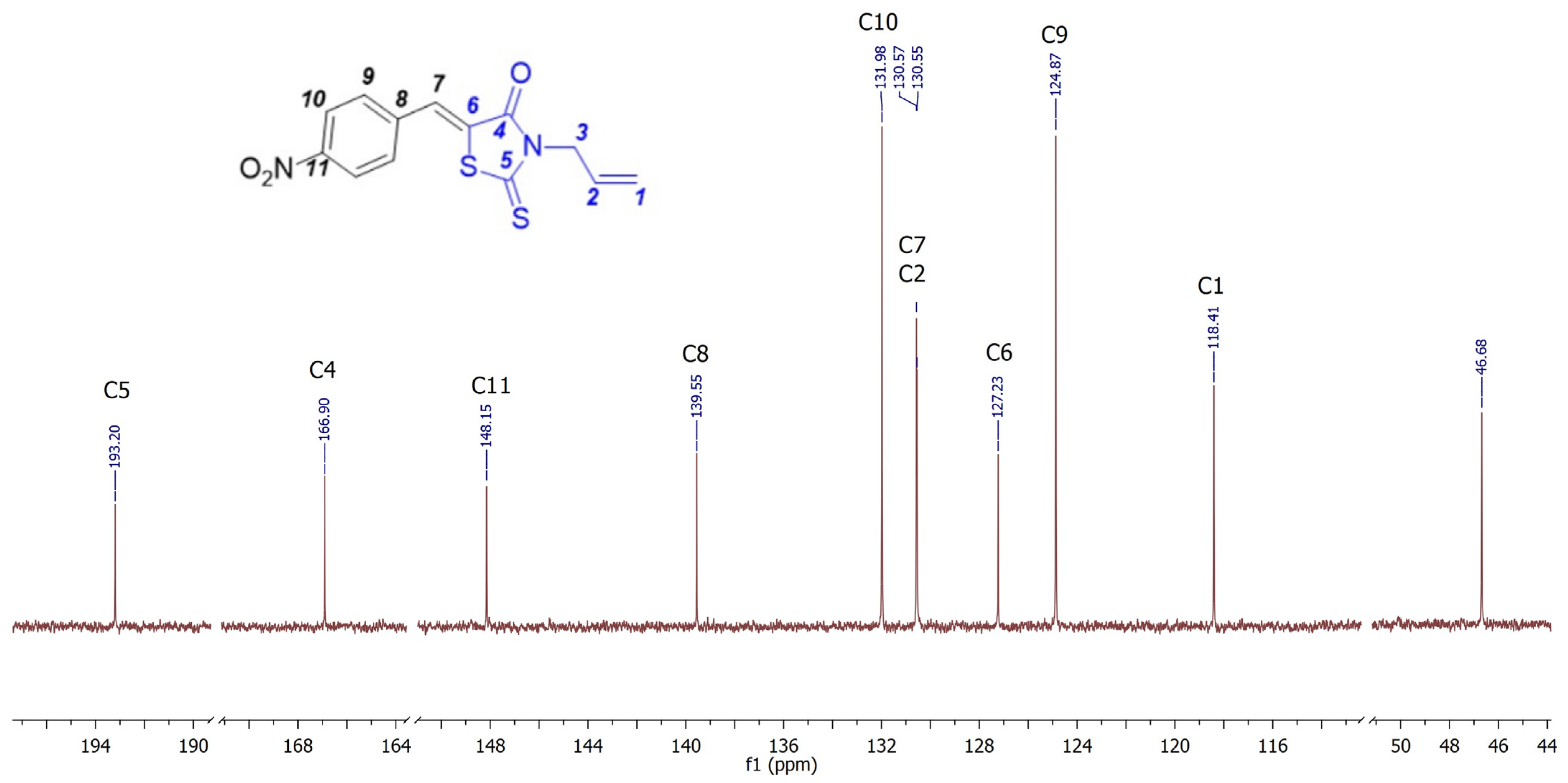
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, F.C.; Bradsher, C.K.; Bond, S.M.; Potter, M. Rhodanine Derivatives. J. Am. Chem. Soc. 1951, 73, 2357–2359. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. Recent Developments with Rhodanine as a Scaffold for Drug Discovery. Expert Opin. Drug Discov. 2017, 12, 1233–1252. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Babapoor, A.; Amani, A.M. A Conceptual Review of Rhodanine: Current Applications of Antiviral Drugs, Anticancer and Antimicrobial Activities. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Bin Ahmad Kamar, A.K.D.; Ju Yin, L.; Tze Liang, C.; Tjin Fung, G.; Avupati, V.R. Rhodanine Scaffold: A Review of Antidiabetic Potential and Structure–Activity Relationships (SAR). Med. Drug Discov. 2022, 15, 100131. [Google Scholar] [CrossRef]
- Naufal, M.; Hermawati, E.; Syah, Y.M.; Hidayat, A.T.; Hidayat, I.W.; Al-Anshori, J. Structure–Activity Relationship Study and Design Strategies of Hydantoin, Thiazolidinedione, and Rhodanine-Based Kinase Inhibitors: A Two-Decade Review. ACS Omega 2024, 9, 4186–4209. [Google Scholar] [CrossRef] [PubMed]
- Bourahla, K.; Guihéneuf, S.; Limanton, E.; Paquin, L.; Le Guével, R.; Charlier, T.; Rahmouni, M.; Durieu, E.; Lozach, O.; Carreaux, F.; et al. Design and Microwave Synthesis of New (5Z) 5-Arylidene-2-Thioxo-1,3-Thiazolinidin-4-One and (5Z) 2-Amino-5-Arylidene-1,3-Thiazol-4(5H)-One as New Inhibitors of Protein Kinase DYRK1A. Pharmaceuticals 2021, 14, 1086. [Google Scholar] [CrossRef] [PubMed]
- Khodair, A.I.; Alzahrani, F.M.; Awad, M.K.; Al-Issa, S.A.; Al-Hazmi, G.H.; Nafie, M.S. Design, Synthesis, Computational Investigations, and Antitumor Evaluation of N-Rhodanine Glycosides Derivatives as Potent DNA Intercalation and Topo II Inhibition against Cancer Cells. ACS Omega 2023, 8, 13300–13314. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.-J.; Li, P.-H.; Wang, J.; Dong, C.-Z.; Zhang, K.; Chen, H.-X.; Du, Z.-Y. Synthesis and Biological Evaluation of Novel Carbazole-Rhodanine Conjugates as Topoisomerase II Inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Szczepański, J.; Tuszewska, H.; Trotsko, N. Anticancer Profile of Rhodanines: Structure–Activity Relationship (SAR) and Molecular Targets—A Review. Molecules 2022, 27, 3750. [Google Scholar] [CrossRef]
- Yin, L.J.; Bin Ahmad Kamar, A.K.D.; Fung, G.T.; Liang, C.T.; Avupati, V.R. Review of Anticancer Potentials and Structure-Activity Relationships (SAR) of Rhodanine Derivatives. Biomed. Pharmacother. 2022, 145, 112406. [Google Scholar] [CrossRef]
- Krátký, M.; Nováčková, K.; Svrčková, K.; Švarcová, M.; Štěpánková, Š. New 3-Amino-2-Thioxothiazolidin-4-One-Based Inhibitors of Acetyl- and Butyryl-Cholinesterase: Synthesis and Activity. Future Med. Chem. 2024, 16, 59–74. [Google Scholar] [CrossRef]
- Krátký, M.; Štěpánková, Š.; Vorčáková, K.; Vinšová, J. Synthesis and in Vitro Evaluation of Novel Rhodanine Derivatives as Potential Cholinesterase Inhibitors. Bioorg. Chem. 2016, 68, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://en.wikipedia.org/wiki/epalrestat (accessed on 9 February 2024).
- Hotta, N.; Sakamoto, N.; Shigeta, Y.; Kikkawa, R.; Goto, Y. Clinical Investigation of Epalrestat, an Aldose Reductase Inhibitor, on Diabetic Neuropathy in Japan: Multicenter Study. J. Diabetes Complicat. 1996, 10, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, P.; Xu, L.; Gao, L.; Li, J.; Piao, H. Discovery of 1,3-Diphenyl-1H-Pyrazole Derivatives Containing Rhodanine-3-Alkanoic Acid Groups as Potential PTP1B Inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Bansal, G.; Singh, S.; Monga, V.; Thanikachalam, P.V.; Chawla, P. Synthesis and Biological Evaluation of Thiazolidine-2,4-Dione-Pyrazole Conjugates as Antidiabetic, Anti-Inflammatory and Antioxidant Agents. Bioorg. Chem. 2019, 92, 103271. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Peng, Y.; Xie, Z.; Wang, J.; Chen, M. Synthesis, α-Glucosidase Inhibition and Molecular Docking Studies of Novel Thiazolidine-2,4-Dione or Rhodanine Derivatives. Med. Chem. Commun. 2017, 8, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Toumi, A.; Boudriga, S.; Hamden, K.; Sobeh, M.; Cheurfa, M.; Askri, M.; Knorr, M.; Strohmann, C.; Brieger, L. Synthesis, Antidiabetic Activity and Molecular Docking Study of Rhodanine-Substitued Spirooxindole Pyrrolidine Derivatives as Novel α-Amylase Inhibitors. Bioorg. Chem. 2021, 106, 104507. [Google Scholar] [CrossRef]
- Dago, C.; Ambeu, C.; Coulibaly, W.-K.; Békro, Y.-A.; Mamyrbékova, J.; Defontaine, A.; Baratte, B.; Bach, S.; Ruchaud, S.; Guével, R.; et al. Synthetic Development of New 3-(4-Arylmethylamino)Butyl-5-Arylidene-Rhodanines under Microwave Irradiation and Their Effects on Tumor Cell Lines and against Protein Kinases. Molecules 2015, 20, 12412–12435. [Google Scholar] [CrossRef]
- Ali Muhammad, S.; Ravi, S.; Thangamani, A. Synthesis and Evaluation of Some Novel N-Substituted Rhodanines for Their Anticancer Activity. Med. Chem. Res. 2016, 25, 994–1004. [Google Scholar] [CrossRef]
- Akhavan, M.; Foroughifar, N.; Pasdar, H.; Bekhradnia, A. Green Synthesis, Biological Activity Evaluation, and Molecular Docking Studies of Aryl Alkylidene 2, 4-Thiazolidinedione and Rhodanine Derivatives as Antimicrobial Agents. Comb. Chem. High Throughput Screen. 2020, 22, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Tomašić, T.; Zidar, N.; Mueller-Premru, M.; Kikelj, D.; Mašič, L.P. Synthesis and Antibacterial Activity of 5-Ylidenethiazolidin-4-Ones and 5-Benzylidene-4,6-Pyrimidinediones. Eur. J. Med. Chem. 2010, 45, 1667–1672. [Google Scholar] [CrossRef]
- Hesse, S. Synthesis of 5-Arylidenerhodanines in L-Proline-Based Deep Eutectic Solvent. Beilstein J. Org. Chem. 2023, 19, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Recent Advances in the Concept of Hard and Soft Acids and Bases. J. Chem. Educ. 1987, 64, 561. [Google Scholar] [CrossRef]
- Moers, F.G.; Bosman, W.P.J.H.; Beurskens, P.T. Crystal Structure of 3-Methylrhodaninecopper(I) Iodide. J. Cryst. Mol. Struct. 1972, 2, 23–29. [Google Scholar] [CrossRef][Green Version]
- Moers, F.G.; Goossens, J.W.M.; Langhout, J.P.M. Rhodanine Complexes of Copper(I), Palladium(II) and Platinum(II). J. Inorg. Nucl. Chem. 1973, 35, 855–859. [Google Scholar] [CrossRef]
- Moers, F.G.; Smits, J.M.M.; Beurskens, P.T. Crystal Structure of Bis-(Rhodanine)Copper(I) Iodide, C6H6CuIN2O2S4. J. Crystallogr. Spectrosc. Res. 1986, 16, 101–106. [Google Scholar] [CrossRef]
- Fabretti, A.C.; Peyronel, G.; Franchini, G.C. Copper(I) Complexes of Rhodanine. Transit. Met. Chem. 1978, 3, 125–127. [Google Scholar] [CrossRef]
- Arar, W.; Khatyr, A.; Knorr, M.; Brieger, L.; Krupp, A.; Strohmann, C.; Efrit, M.L.; Ben Akacha, A. Synthesis, Crystal Structures and Hirshfeld Analyses of Phosphonothioamidates (EtO)2P(=O)C(=S)N(H)R (R = Cy, Bz) and Their Coordination on CuI and HgX2 (X = Br, I). Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 845–858. [Google Scholar] [CrossRef]
- Hameau, A.; Guyon, F.; Knorr, M.; Enescu, M.; Strohmann, C. Self-Assembly of Dithiolene-Based Coordination Polymers of Mercury(II): Dithioether versus Thiocarbonyl Bonding. Monatsh. Chem. 2006, 137, 545–555. [Google Scholar] [CrossRef]
- Guyon, F.; Hameau, A.; Khatyr, A.; Knorr, M.; Amrouche, H.; Fortin, D.; Harvey, P.D.; Strohmann, C.; Ndiaye, A.L.; Huch, V.; et al. Syntheses, Structures, and Photophysical Properties of Mono- and Dinuclear Sulfur-Rich Gold(I) Complexes. Inorg. Chem. 2008, 47, 7483–7492. [Google Scholar] [CrossRef][Green Version]
- Hameau, A.; Guyon, F.; Khatyr, A.; Knorr, M.; Strohmann, C. 4,5-Bis(Methylthio)-1,3-Dithiole-2-Thione, a Versatile Sulphur-Rich Building Block for the Self-Assembly of Cu(I) and Ag(I) Coordination Polymers: Dithioether versus Thiocarbonyl Bonding. Inorg. Chim. Acta 2012, 388, 60–70. [Google Scholar] [CrossRef]
- Arar, W.; Viau, L.; Jourdain, I.; Knorr, M.; Strohmann, C.; Scheel, R.; Ben Akacha, A. Synthesis of Catena-Bis(μ-Bromo)-(O-Methyl-N-Phenylthiocarbamate)-Dicopper(I) and Its Reactivity towards PAr3 (Ar = Ph, p-Tol). Molbank 2023, 2023, M1655. [Google Scholar] [CrossRef]
- Gouveia, F.L.; De Oliveira, R.M.B.; De Oliveira, T.B.; Da Silva, I.M.; Do Nascimento, S.C.; De Sena, K.X.F.R.; De Albuquerque, J.F.C. Synthesis, Antimicrobial and Cytotoxic Activities of Some 5-Arylidene-4-Thioxo-Thiazolidine-2-Ones. Eur. J. Med. Chem. 2009, 44, 2038–2043. [Google Scholar] [CrossRef] [PubMed]
- El Ajlaoui, R.; Ouafa, A.; Mojahidi, S.; El Ammari, L.; Saadi, M.; El Mostapha, R. Unexpected Synthesis of Novel 3-Allyl-5-(Arylidene)-2-Thioxo-Thiazolidin-4-Ones in Reactions of 3-Allylrhodanine with 2-Arylidene-4-Methyl-5-Oxopyrazolidinium Ylides. Synth. Commun. 2015, 45, 2035–2042. [Google Scholar] [CrossRef]
- Momose, Y.; Meguro, K.; Ikeda, H.; Hatanaka, C.; Oi, S.; Sohda, T. Studies on Antidiabetic Agents. X. Synthesis and Biological Activities of Pioglitazone and Related Compounds. Chem. Pharm. Bull. 1991, 39, 1440–1445. [Google Scholar] [CrossRef]
- El Ajlaoui, R.; Rakib, E.M.; Mojahidi, S.; Saadi, M.; El Ammari, L. (Z)-3-Allyl-5-(3-Methoxybenzylidene)-2-Sulfanylidene-1,3-Thiazolidin-4-One. IUCrData 2016, 1, x160052. [Google Scholar] [CrossRef]
- El Ajlaoui, R.; Belkhouya, N.; Rakib, E.M.; Mojahidi, S.; Saadi, M.; El Ammari, L. (Z)-3-Allyl-5-(4-Fluorobenzylidene)-2-Sulfanylidenethiazolidin-4-one. IUCrData 2016, 1, x161236. [Google Scholar] [CrossRef]
- El Ajlaoui, R.; Rakib, E.M.; Mojahidi, S.; Saadi, M.; El Ammari, L. Crystal Structure of (Z)-3-Allyl-5-(4-Chlorobenzylidene)-2-Sulfanylidene-1,3-Thiazolidin-4-one. Acta Crystallogr. Sect. E Crystallogr. Commun. 2015, 71, o1012. [Google Scholar] [CrossRef]
- Germain, G.; Piret, P.; Van Meersche, M.; De Kerf, J. Structure d’une Mérocyanine: C10H12S3N2O. Acta Crystallogr. 1962, 15, 373–382. [Google Scholar] [CrossRef]
- El Ajlaoui, R.; Rakib, E.M.; Mojahidi, S.; Saadi, M.; El Ammari, L. Crystal Structure of (E)-3-Allyl-2-Sulfanylidene-5-[(Thiophen-2-Yl)Methylidene]Thiazolidin-4-one. Acta Crystallogr. Sect. E Crystallogr. Commun. 2015, 71, o433–o434. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
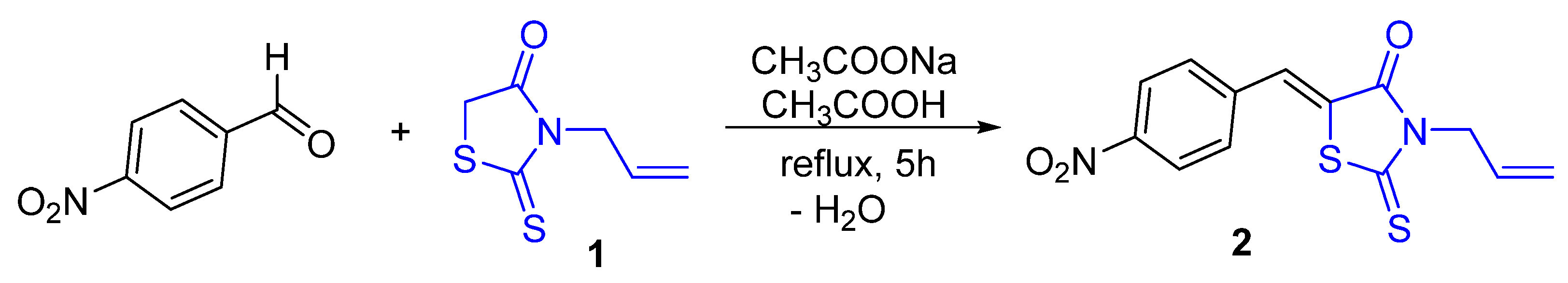
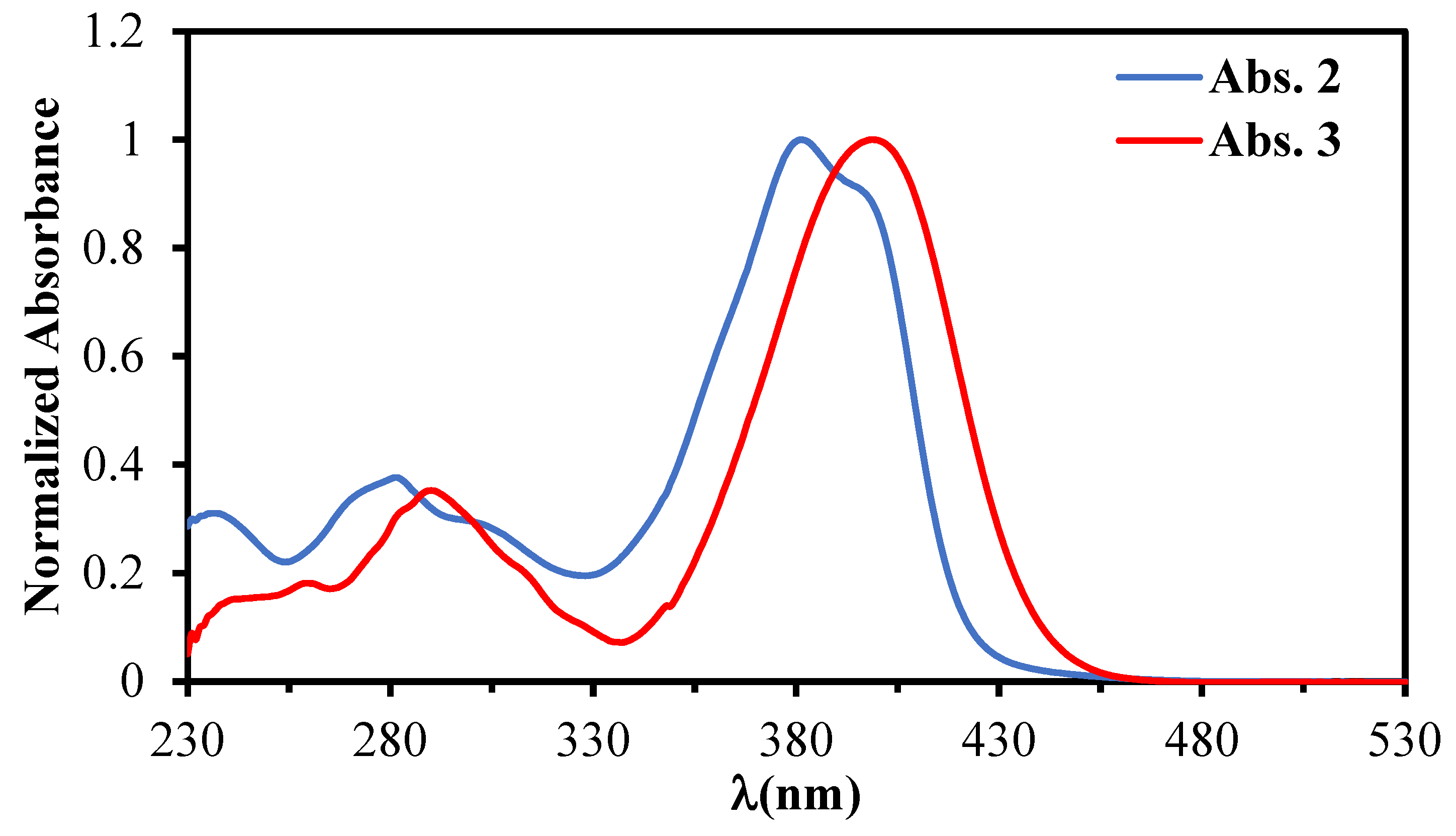
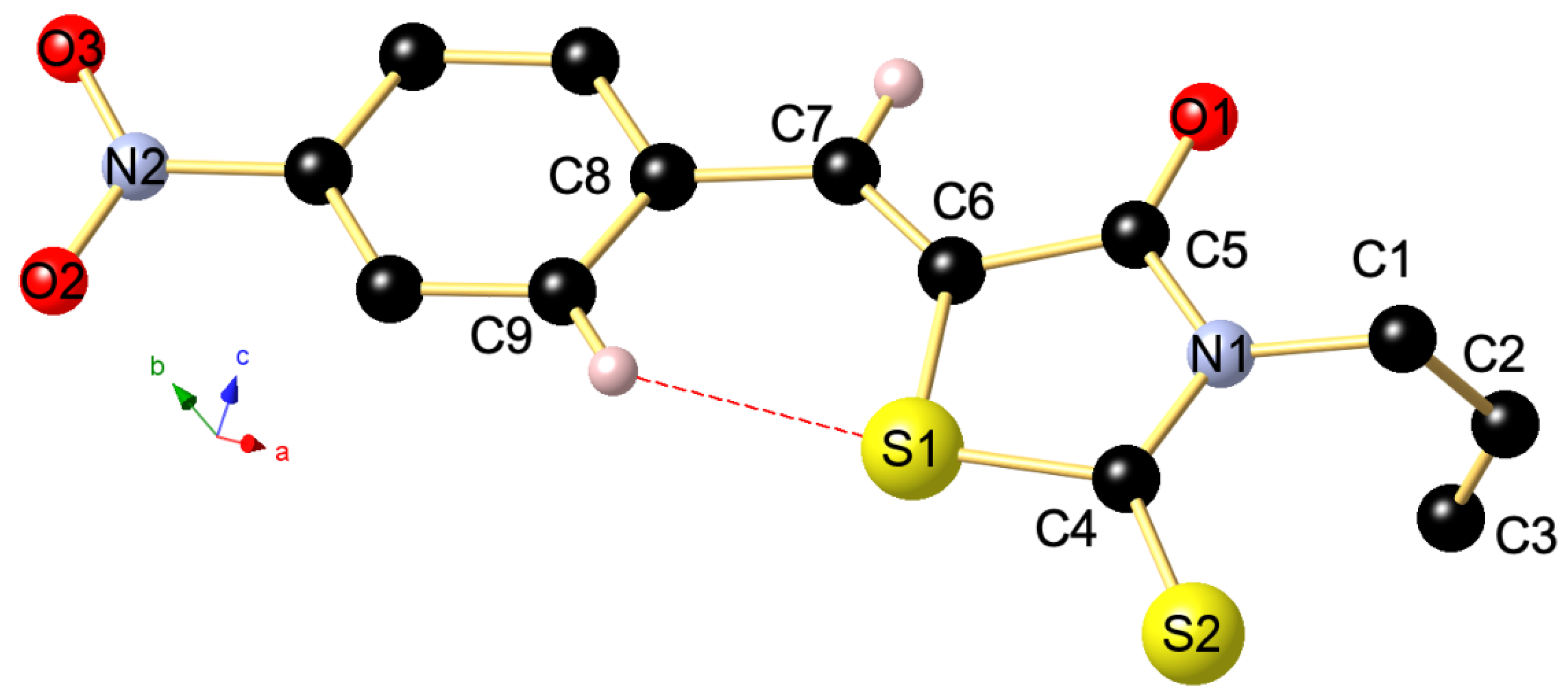
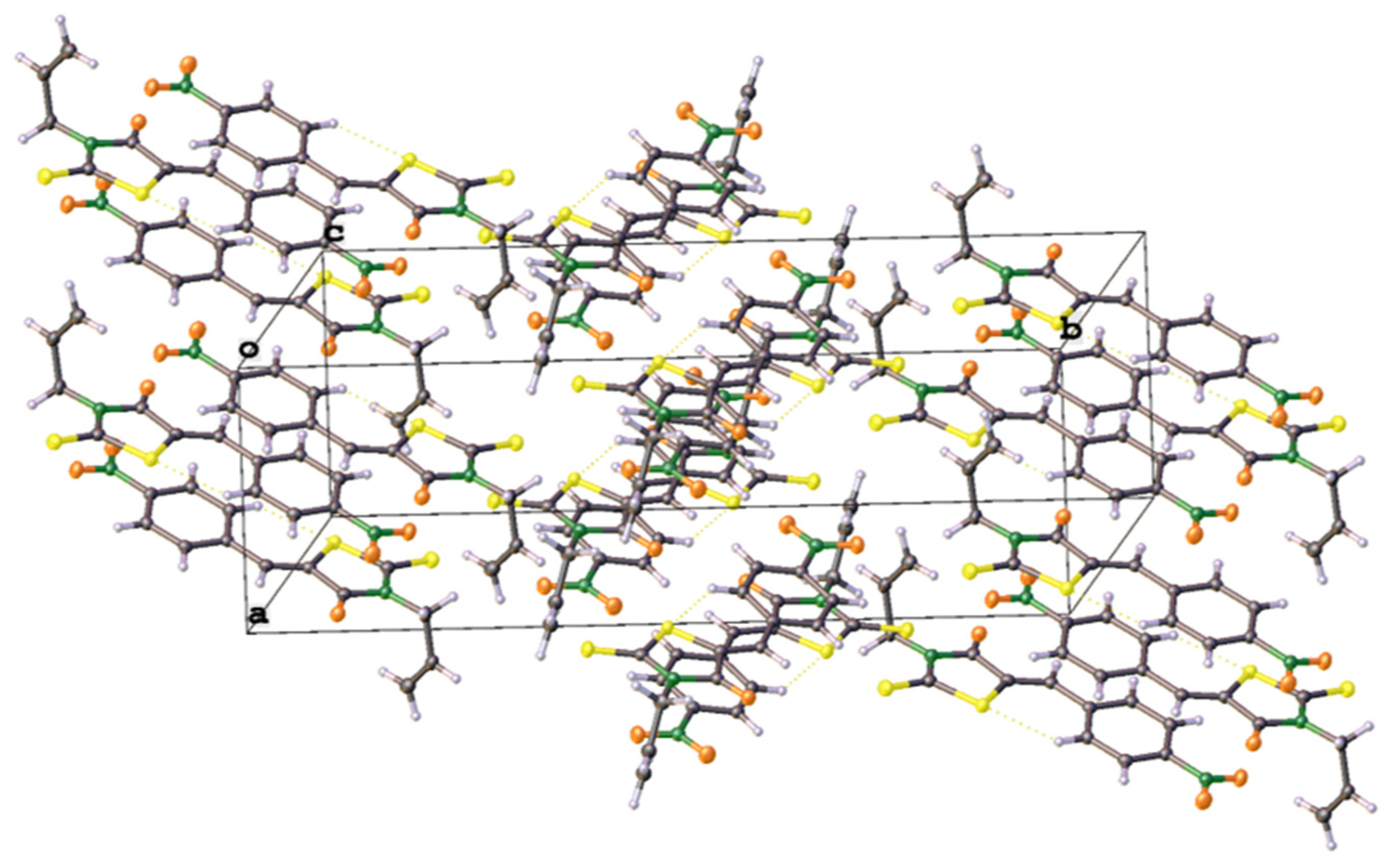

| Comp. | Absorption: λabs nm (ε × 10−3M−1cm−1) |
|---|---|
| 2 | 239 (5.5), 281 (6.7), 303 sh (4.8), 381 (17.9), 399 sh (16.0) |
| 3 | 242 (2.8), 262 (3.2), 294 (6.1), 313 sh (3.6), 399 (18.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, B.; Jourdain, I.; Knorr, M.; Boudriga, S.; Strohmann, C.; Schrimpf, T. Synthesis of (Z)-3-Allyl-5-(4-nitrobenzylidene)-2-sulfanylidene-1,3-thiazolidin-4-one and Determination of Its Crystal Structure. Molbank 2024, 2024, M1783. https://doi.org/10.3390/M1783
Moreno B, Jourdain I, Knorr M, Boudriga S, Strohmann C, Schrimpf T. Synthesis of (Z)-3-Allyl-5-(4-nitrobenzylidene)-2-sulfanylidene-1,3-thiazolidin-4-one and Determination of Its Crystal Structure. Molbank. 2024; 2024(1):M1783. https://doi.org/10.3390/M1783
Chicago/Turabian StyleMoreno, Bastien, Isabelle Jourdain, Michael Knorr, Sarra Boudriga, Carsten Strohmann, and Tobias Schrimpf. 2024. "Synthesis of (Z)-3-Allyl-5-(4-nitrobenzylidene)-2-sulfanylidene-1,3-thiazolidin-4-one and Determination of Its Crystal Structure" Molbank 2024, no. 1: M1783. https://doi.org/10.3390/M1783
APA StyleMoreno, B., Jourdain, I., Knorr, M., Boudriga, S., Strohmann, C., & Schrimpf, T. (2024). Synthesis of (Z)-3-Allyl-5-(4-nitrobenzylidene)-2-sulfanylidene-1,3-thiazolidin-4-one and Determination of Its Crystal Structure. Molbank, 2024(1), M1783. https://doi.org/10.3390/M1783








