Abstract
Herein, we describe the synthesis of 3-(2-chloroethoxy)-1-(4-methoxyphenyl)-1H-pyrazole-4-carbaldehyde via the Vilsmeier-Haack reaction. The structure of this previously unreported compound is thoroughly elucidated through NMR, FT-IR spectroscopy and HRMS spectrometry.
1. Introduction
The Vilsmeier-Haack reaction is a useful tool for the formylation of aromatic and heterocyclic compounds, such as pyrroles, imidazoles, pyrazoles, etc. [1]. It has been shown that variously substituted pyrazole derivatives can be transformed into condensed pyrazolo[3,4-b]pyridines, pyrazolo[1,5-a]-, and pyrazolo[3,4-d]pyrimidines through the Vilsmeier-Haack reaction [2,3]. Our group has successfully employed the Vilsmeier-Haack formylation reaction to obtain various pyrazole-4-carbaldehydes from commercially available 1-phenyl-1H-pyrazol-3-ol and utilized them to prepare fluorescent sensors [4] and biologically active compounds [5,6,7]. Additionally, it has been reported that the products of the Vilsmeier-Haack formylation reaction could be versatile scaffolds in the synthesis of materials for solar cells [8,9], organic light-emitting diodes [10,11], and natural products [12,13,14].
Usually, dimethylformamide and phosphorus trichloride are utilized to form the Vilsmeier reagent [15]. The latter was shown to participate in chlorination as well as chloroformylation reactions [16]. Compounds containing a 2-chloroethoxy chain are of particular interest, as they can be readily modified through a nucleophilic substitution reaction with various amines to furnish aminoalkoxy-functionalized potential anti-cancer, neuroprotective, or biological imaging agents [17,18,19]. 2-Amino-6-(2-chloroethoxy)benzothiazole was used in the preparation of cationic azo dyes [20], while 2-chloroethoxyacetaldehyde has been revealed to participate in an organocatalytic asymmetric direct cross-aldol reaction with aromatic aldehydes to yield chiral dioxanes and morpholines, which often possess various biological activities [21].
In this work, we employ the Vilsmeier-Haack reaction to achieve dual functionalization of 3-(2-methoxyethoxy)-1-(4-methoxyphenyl)-1H-pyrazole (1), giving rise to 3-(2-chloroethoxy)-1-(4-methoxyphenyl)-1H-pyrazole-4-carbaldehyde (2). The structure of the newly synthesized compound 2 was elucidated through NMR, FT-IR spectroscopy, and HRMS spectrometry.
2. Results and Discussion
Examples of structural modifications leading to the formation of haloalkoxy chains are relatively scarce in the scientific literature. The 2-ethoxyethanol groups can be converted to the more reactive 2-bromoethoxy chains using phosphorus bromide in toluene [22]. Meanwhile, the 2-chloroethoxy chain can be inserted through the palladium-catalysed chloroethoxylation reaction of aryl chlorides with tetrakis(2-chloroethoxy)borate, with good tolerance towards different existing functional groups in the molecule, including formyl [23]. Another method for introducing a chloroethoxy group entails reacting phenols with 1,2-dichloroethane in the presence of potassium carbonate as a base [24]. The possibility of the methoxy group cleavage and simultaneous replacement with a chlorine atom in a methoxyethoxy chain, in the presence of pyridine and phosphorus oxychloride, or phosphorus oxychloride/diethylamine in toluene, has been only briefly described in the synthesis of quinazoline-based pharmaceuticals [25,26].
Since phosphorus oxychloride is also known to participate in the Vilsmeier-Haack formylation reaction in conjunction with dimethylformamide, we ought to perform a dual functionalization, namely, to simultaneously formylate, and chlorinate 3-(2-methoxyethoxy)-1-(4-methoxyphenyl)-1H-pyrazole (1) to obtain a (2-chloroethoxysubstituted)pyrazole carbaldehyde 2. The starting material 1 was readily prepared from 1-(4-methoxyphenyl)-3-hydroxy-1H-pyrazole adopting a previously published synthetic method [27]. It was then subjected to the reaction with the Vilsmeier reagent, which has been pre-formed from equimolar amounts of phosphorus oxychloride and dimethylformamide at −10 °C, and then the reaction mixture was heated to 70 °C and stirred for 24 h. After column chromatography, the target compound 2 (Figure 1) was obtained in a moderate yield of 48%.
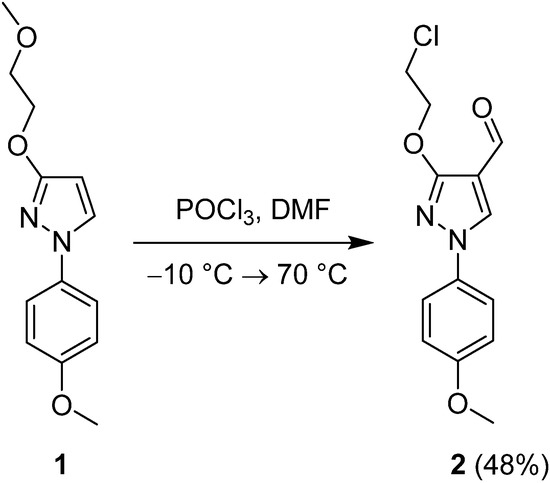
Figure 1.
Synthesis of compound 2.
The structure of compound 2 was thoroughly elucidated through NMR spectroscopy (Figure 2). In the 1H NMR spectrum of compound 2 (Figure S1), only one singlet of methoxy group protons appeared at δ 3.85 ppm. Then, according to the 1H-13C heteronuclear single quantum coherence (HSQC) spectrum, these protons had a one-bond correlation with the carbon, which resonated at δ 55.6 ppm. Furthermore, the 1H-13C heteronuclear multiple bond correlation (HMBC) spectrum revealed a correlation between these methoxy group protons and the quaternary carbon at δ 158.9 ppm (phenyl C-4′). The 1H-1H NOESY spectral data revealed that the methoxy group protons exhibited NOEs with the neighboring phenyl group 3′(5′)-H protons (δ 6.95−7.00 ppm), confirming their proximity in space (Figure S3). Thus, it was concluded that only a cleavage of the methoxy group in the 2-methoxyethoxy chain had occurred. The DEPT-135 spectrum clearly showed the negative -CH2 peaks at δ 68.9 and 41.5 ppm (Figure S4); the latter was significantly upfield due to the absence of a neighboring methoxy group. Finally, the most downfield 1H and 13C NMR signals were easily attributed to the formyl moiety via 1H-13C HSQC and HMBC spectral data (Figure S5). The 15N NMR data were obtained through the 1H-15N HMBC experiment where the pyrazole 5-H proton (singlet, δ 8.15 ppm) exhibited long-range correlations with neighboring N-1 “pyrrole-like” (δ −179.0 ppm) and N-2 “pyridine-like” (δ −117.0 ppm) nitrogen atoms (Figure S6).
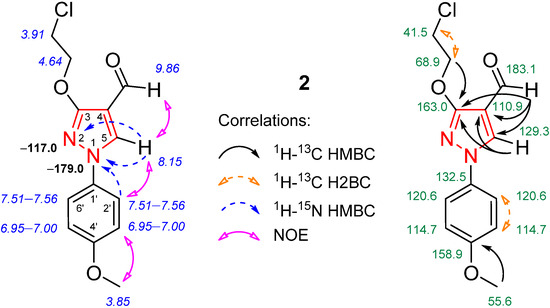
Figure 2.
Relevant 1H-13C HMBC, 1H-13C H2BC, 1H-15N HMBC, and 1H-1H NOESY correlations, as well as 1H NMR (in italics), 15N NMR (in bold), and 13C NMR chemical shifts of compound 2.
The replacement of the methoxy group in the methoxyethoxy chain with a chlorine atom was confirmed by high-resolution mass spectrometry (HRMS) data, as the HRMS spectrum showed a molecular ion [M + H]+ at m/z 281.0691 (calculated for C13H14ClN2O3, 281.0687) and the predominant sodium adduct [M + Na]+ at m/z 303.0508 (see Figure S7). A characteristic, intense formyl group peak in the FT-IR spectrum of compound 2 is situated at 1667 cm−1.
3. Materials and Methods
All chemicals and solvents were purchased from commercial suppliers and used without further purification. NMR spectra were recorded in CDCl3 solutions at 25 °C on a Bruker Avance III 700 (700 MHz for 1H, 176 MHz for 13C, 71 MHz for 15N) spectrometer equipped with a 5 mm TCI 1H-13C/15N/D z-gradient cryoprobe (Bruker BioSpin AG, Fällanden, Switzerland) and processed using TopSpin 3.6.4 and MestReNova 11.0 software. The chemical shifts, expressed in ppm, were relative to tetramethylsilane (TMS). The 15N NMR (1H-15N HMBC) spectrum was referenced to neat, external nitromethane (coaxial capillary). FT-IR spectrum was collected using the ATR method on a Bruker Vertex 70v spectrometer (Bruker Optik GmbH, Ettlingen, Germany) with an integrated Platinum ATR accessory and processed using OPUS 7.2 software. The melting point was determined in an open capillary tube with a Buchi M-565 apparatus (Büchi Labortechnik AG, Flawil, Switzerland) and was uncorrected (temperature gradient—2 °C/min). High-resolution mass spectrometry (HRMS) spectrum was obtained in ESI mode with a Bruker MicrOTOF-Q III spectrometer (Bruker Daltonik GmbH, Bremen, Germany) and processed with Bruker Compass DataAnalysis 4.1 software. Reaction progress was monitored by TLC analysis on Macherey-Nagel™ ALUGRAM® Xtra SIL G/UV254 plates (Macherey-Nagel GmbH & Co., Düren, Germany). TLC plates were visualized with UV light (wavelengths 254 and 365 nm). Compounds were purified by flash chromatography in a glass column (stationary phase—silica gel 60, 0.063–0.200 mm, 70–230 mesh ASTM, Merck KGaA, Darmstadt, Germany).
Synthesis of 3-(2-chloroethoxy)-1-(4-methoxyphenyl)-1H-pyrazole-4-carbaldehyde (2): Phosphorus oxychloride (40 mmol, 2.5 mL, 4 eq.) was added dropwise into dry dimethylformamide (40 mmol, 2.1 mL, 4 eq.) under Ar at −10 °C. The mixture was stirred at −10 °C until the viscous, white Vilsmeier reagent was formed. Then, 3-(2-methoxyethoxy)-1-(4-methoxyphenyl)-1H-pyrazole (1) (6.7 mmol, 1680 mg, 1 eq.) was dissolved in dry dimethylformamide (5 mL) and added dropwise into the Vilsmeier reagent at r.t. The reaction temperature was subsequently raised to 70 °C and maintained for 24 h. Subsequently, the reaction mixture was chilled, poured into ice water (200 mL), and basified with solid Na2CO3 and NaOH (pH > 10). The resulting precipitate was filtered and purified by column chromatography on silica gel (eluent—EtOAc/Hex 1/3 to 1/1 v/v) to give 2 (Rf = 0.22, EtOAc/Hex 3/1 v/v) as a white solid (907 mg, 48% yield). m.p. = 106–107 °C; IR (neat) νmax 3127, 3101, 2940, 2826, 2748 (CHarom, CHaliph), 1667 (C=O), 1560, 1516, 1499, 1367, 1304, 1246, 1228, 1177, 1024, 944, 825 (C=C, CH3, CH2 bending, C-O, C-N, C-Harom oop bending) cm−1; 1H NMR (700 MHz, CDCl3): δ 9.86 (s, 1H, -CHO), 8.15 (s, 1H, Pyr 5-H), 7.56–7.51 (m, 2H, Ph 2,6-H), 7.00–6.95 (m, 2H, Ph 3,5-H), 4.64 (t, J = 5.9 Hz, 2H, -OCH2CH2Cl), 3.91 (t, J = 5.9 Hz, 2H, -OCH2CH2Cl), 3.85 (s, 3H, -OCH3). 13C NMR (176 MHz, CDCl3): δ 183.1 (-CHO), 163.0 (Pyr C-3), 158.9 (Ph C-4), 132.5 (Ph C-1), 129.3 (Pyr C-5), 120.6 (Ph C-2,6), 114.7 (Ph C-3,5), 110.9 (Pyr C-4), 68.9 (-OCH2CH2Cl), 55.6 (-OCH3), 41.5 (-OCH2CH2Cl). 15N NMR (71 MHz, CDCl3): δ −179.0 (N-1), −117.0 (N-2). HRMS (ESI-TOF) for C13H13ClN2NaO3 ([M + Na]+): calcd. 303.0507; found 303.0508, Δ = −0.3 ppm.
Supplementary Materials
2D MDL molfile of Compound 2, Figure S1: 1H NMR spectrum of compound 2; Figure S2: 13C NMR spectrum of compound 2; Figure S3: 1H-1H NOESY spectrum of compound 2; Figure S4: 13C NMR/DEPT 135 spectra of compound 2; Figure S5: The overlaid 1H-13C HSQC/HMBC NMR spectra of compound 2; Figure S6: 1H-15N HMBC spectrum of compound 2; Figure S7: FT-IR spectrum of compound 2; Figure S8: HRMS spectrum of compound 2.
Author Contributions
Conceptualization, A.B. and A.Š.; methodology, A.B. and A.Š.; formal analysis, G.V.; investigation, G.V. and A.B.; resources, A.Š.; data curation, A.B.; writing—original draft preparation, G.V.; writing—review and editing, A.Ž., N.K., A.B. and A.Š.; visualization, G.V. and A.B.; supervision, N.K.; project administration, A.Š.; funding acquisition, A.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Council of Lithuania (No. S-MIP-23-51).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank Miglė Dagilienė and Vaida Milišiūnaitė for the HRMS measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chahal, M.; Dhillon, S.; Rani, P.; Kumari, G.; Aneja, D.K.; Kinger, M. Unravelling the Synthetic and Therapeutic Aspects of Five, Six and Fused Heterocycles Using Vilsmeier–Haack Reagent. RSC Adv. 2023, 13, 26604–26629. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, J.; Diaz, Y.; Insuasty, B.; Abonıa, R.; Nogueras, M.; Cobo, J. Preparation of 6-Chloropyrazolo[3,4-b]Pyridine-5-Carbaldehydes by Vilsmeier–Haack Reaction and Its Use in the Synthesis of Heterocyclic Chalcones and Dipyrazolopyridines. Tetrahedron Lett. 2010, 51, 2928–2930. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Insuasty, B.; Abonía, R.; Nogueras, M.; Cobo, J. Regioselective Formylation of Pyrazolo[3,4-b]Pyridine and Pyrazolo[1,5-a]Pyrimidine Systems Using Vilsmeier–Haack Conditions. Tetrahedron Lett. 2008, 49, 2689–2691. [Google Scholar] [CrossRef]
- Kazlauskas, K.; Kreiza, G.; Arbačiauskienė, E.; Bieliauskas, A.; Getautis, V.; Šačkus, A.; Juršėnas, S. Morphology and Emission Tuning in Fluorescent Nanoparticles Based on Phenylenediacetonitrile. J. Phys. Chem. C 2014, 118, 25261–25271. [Google Scholar] [CrossRef]
- Varvuolytė, G.; Malina, L.; Bieliauskas, A.; Hošíková, B.; Simerská, H.; Kolářová, H.; Kleizienė, N.; Kryštof, V.; Šačkus, A.; Žukauskaitė, A. Synthesis and Photodynamic Properties of Pyrazole-Indole Hybrids in the Human Skin Melanoma Cell Line G361. Dye. Pigment. 2020, 183, 108666. [Google Scholar] [CrossRef]
- Milišiūnaitė, V.; Arbačiauskienė, E.; Řezníčková, E.; Jorda, R.; Malínková, V.; Žukauskaitė, A.; Holzer, W.; Šačkus, A.; Kryštof, V. Synthesis and Anti-Mitotic Activity of 2,4- or 2,6-Disubstituted- and 2,4,6-Trisubstituted-2H-Pyrazolo [4,3-c]Pyridines. Eur. J. Med. Chem. 2018, 150, 908–919. [Google Scholar] [CrossRef]
- Razmienė, B.; Řezníčková, E.; Dambrauskienė, V.; Ostruszka, R.; Kubala, M.; Žukauskaitė, A.; Kryštof, V.; Šačkus, A.; Arbačiauskienė, E. Synthesis and Antiproliferative Activity of 2,4,6,7-Tetrasubstituted-2H-Pyrazolo[4,3-c]Pyridines. Molecules 2021, 26, 6747. [Google Scholar] [CrossRef]
- Urnikaitė, S.; Malinauskas, T.; Bruder, I.; Send, R.; Gaidelis, V.; Sens, R.; Getautis, V. Organic Dyes with Hydrazone Moieties: A Study of Correlation between Structure and Performance in the Solid-State Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 7832–7843. [Google Scholar] [CrossRef]
- Igci, C.; Paek, S.; Rakstys, K.; Kanda, H.; Shibayama, N.; Jankauskas, V.; Roldán-Carmona, C.; Kim, H.; Asiri, A.M.; Nazeeruddin, M.K. D–π–A-Type Triazatruxene-Based Dopant-Free Hole Transporting Materials for Efficient and Stable Perovskite Solar Cells. Sol. RRL 2020, 4, 2000173. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, C.Y.; Kang, H.; Jeong, J.-E.; Woo, H.Y.; Cho, M.J.; Park, S.; Choi, D.H. Universal Polymeric Bipolar Hosts for Highly Efficient Solution-Processable Blue and Green Thermally Activated Delayed Fluorescence OLEDs. J. Mater. Chem. C Mater. 2020, 8, 16048–16056. [Google Scholar] [CrossRef]
- Mai, R.; Wu, X.; Jiang, Y.; Meng, Y.; Liu, B.; Hu, X.; Roncali, J.; Zhou, G.; Liu, J.-M.; Kempa, K.; et al. An Efficient Multi-Functional Material Based on Polyether-Substituted Indolocarbazole for Perovskite Solar Cells and Solution-Processed Non-Doped OLEDs. J. Mater. Chem. A Mater. 2019, 7, 1539–1547. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, J.; Fan, A.; Cui, Y.; Jia, Y. Total Synthesis of Lamellarins D, H, and R and Ningalin B. Org. Lett. 2011, 13, 312–315. [Google Scholar] [CrossRef]
- Mathew, P.; Asokan, C.V. Cyclization of Functionalized Ketene-N,S-Acetals to Substituted Pyrroles: Applications in the Synthesis of Marine Pyrrole Alkaloids. Tetrahedron Lett. 2005, 46, 475–478. [Google Scholar] [CrossRef]
- Boudreault, J.; Lévesque, F.; Bélanger, G. Studies toward Total Synthesis of (±)-Caldaphnidine C via One-Pot Sequential Intramolecular Vilsmeier–Haack and Azomethine Ylide 1,3-Dipolar Cycloaddition. J. Org. Chem. 2016, 81, 9247–9268. [Google Scholar] [CrossRef]
- Abdelhamid, I.A.; Shaaban, M.R.; Elwahy, A.H.M. Applications of the Vilsmeier Reaction in Heterocyclic Chemistry. In Advances in Heterocyclic Chemistry; Academic Press: Cambridge, MA, USA, 2022; Volume 136, pp. 171–223. [Google Scholar]
- Su, W.; Weng, Y.; Jiang, L.; Yang, Y.; Zhao, L.; Chen, Z.; Li, Z.; Li, J. Recent Progress in the Use of Vilsmeier-Type Reagents. Org. Prep. Proced. Int. 2010, 42, 503–555. [Google Scholar] [CrossRef]
- Luo, G.; Chen, M.; Lyu, W.; Zhao, R.; Xu, Q.; You, Q.; Xiang, H. Design, Synthesis, Biological Evaluation and Molecular Docking Studies of Novel 3-Aryl-4-Anilino-2H-Chromen-2-One Derivatives Targeting ERα as Anti-Breast Cancer Agents. Bioorg. Med. Chem. Lett. 2017, 27, 2668–2673. [Google Scholar] [CrossRef]
- Abdellatif, K.R.A.; Velázquez, C.A.; Huang, Z.; Chowdhury, M.A.; Knaus, E.E. Triaryl (Z)-Olefins Suitable for Radiolabeling with Iodine-124 or Fluorine-18 Radionuclides for Positron Emission Tomography Imaging of Estrogen Positive Breast Tumors. Bioorg. Med. Chem. Lett. 2011, 21, 1195–1198. [Google Scholar] [CrossRef]
- Linciano, P.; Sorbi, C.; Rossino, G.; Rossi, D.; Marsala, A.; Denora, N.; Bedeschi, M.; Marino, N.; Miserocchi, G.; Dondio, G.; et al. Novel S1R Agonists Counteracting NMDA Excitotoxicity and Oxidative Stress: A Step Forward in the Discovery of Neuroprotective Agents. Eur. J. Med. Chem. 2023, 249, 115163. [Google Scholar] [CrossRef]
- Deligeorgiev, T.G.; Simov, D. Preparation of Cationic Azo Dyes Derived from 2-Amino-6-(2-Chloroethoxy)Benzothiazole and 2-Amino-4-(2-Hydroxyethoxy)Benzothiazole. Dye. Pigm. 1998, 38, 115–125. [Google Scholar] [CrossRef]
- Sawant, R.T.; Stevenson, J.; Odell, L.R.; Arvidsson, P.I. Organocatalytic Asymmetric Cross-Aldol Reaction of 2-Chloroethoxy Acetaldehyde: Diversity-Oriented Synthesis of Chiral Substituted 1,4-Dioxanes and Morpholines. Tetrahedron Asymmetry 2013, 24, 134–141. [Google Scholar] [CrossRef]
- Stoffelen, C.; Staltari-Ferraro, E.; Huskens, J. Effects of the Molecular Weight and the Valency of Guest-Modified Poly(Ethylene Glycol)s on the Stability, Size and Dynamics of Supramolecular Nanoparticles. J. Mater. Chem. B 2015, 3, 6945–6952. [Google Scholar] [CrossRef] [PubMed]
- Pethő, B.; Vangel, D.; Csenki, J.T.; Zwillinger, M.; Novák, Z. Palladium Catalyzed Chloroethoxylation of Aromatic and Heteroaromatic Chlorides: An Orthogonal Functionalization of a Chloroethoxy Linker. Org. Biomol. Chem. 2018, 16, 4895–4899. [Google Scholar] [CrossRef] [PubMed]
- Jawabrah Al-Hourani, B.; El-Barghouthi, M.I.; Al-Awaida, W.; McDonald, R.; Fattash, I.A.; El Soubani, F.; Matalka, K.; Wuest, F. Biomolecular Docking, Synthesis, Crystal Structure, and Bioassay Studies of 1-[4-(2-Chloroethoxy)Phenyl]-5-[4-(Methylsulfonyl)Phenyl]-1H-Tetrazole and 2-(4-(5-(4-(Methylsulfonyl)Phenyl)-1H-Tetrazol-1-Yl)Phenoxy)Ethyl Nitrate. J. Mol. Struct. 2020, 1202, 127323. [Google Scholar] [CrossRef]
- Zhang, G.; Zha, L. Isolation of Highly Pure Erlotinib Hydrochloride by Recrystallization after Nucleophilic Substitution of an Impurity with Piperazine. Res. Chem. Intermed. 2013, 39, 2303–2309. [Google Scholar] [CrossRef]
- Wang, Y.; Metcalf, C.A.I.; Shakespeare, W.C.; Sawyer, T.K.; Bohacek, R. Novel Quinazolines and Uses Thereof. WO03000188A2, 8 January 2003. [Google Scholar]
- Urbonavičius, A.; Fortunato, G.; Ambrazaitytė, E.; Plytninkienė, E.; Bieliauskas, A.; Milišiūnaitė, V.; Luisi, R.; Arbačiauskienė, E.; Krikštolaitytė, S.; Šačkus, A. Synthesis and Characterization of Novel Heterocyclic Chalcones from 1-Phenyl-1H-Pyrazol-3-Ol. Molecules 2022, 27, 3752. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).