Abstract
A novel oxylipin, okeanoate (1), was isolated from the Okinawan cyanobacterium Okeania hirsuta. The structure of 1 was elucidated based on spectroscopic data including 1D and 2D NMR, as well as high-resolution mass spectrometry. This is the first oxylipin with a dimethylsulfonium moiety in the middle of the hydrocarbon chain.
1. Introduction
Oxylipins are a group of oxygenated natural products formed from fatty acids [1]. It has been reported that many oxylipins perform physiologically important activities in many organisms [2]. Cyanobacteria are known to produce numerous bioactive substances and are recognized as valuable biochemical resources [3,4]. A few oxylipins have been reported as natural products from cyanobacteria so far [5,6,7,8,9,10]. Our research group has recently reported two known isolated oxylipins, malyngic acid, 15,16-dihydro malyngic acid, and a new oxylipin, okeanic acid–A [10], from the marine cyanobacterium Okeania hirsuta. In this study, further research on the cyanobacterium O. hirsuta extracts led to the isolation of a novel oxylipin, (10E,15Z)-12-(dimethylsulfonio)-9,13-dihydroxyoctadeca-10,15-dienoate (1, Figure 1). Only a few oxylipins with a dimethylsulfonium group at the end of the hydrocarbon chain have been reported from nature [11,12]. To the best of our knowledge, there are no reports of oxylipins having a dimethylsulfonium group in the middle of the hydrocarbon chain so far. This is the first finding of an oxylipin with a dimethylsulfonium moiety in the middle of the hydrocarbon chain from nature. The compound (1) was designated as okeanoate.
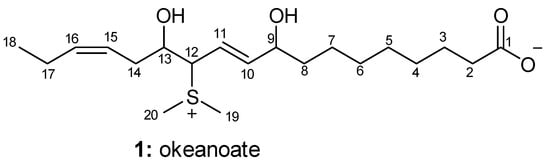
Figure 1.
Structure of okeanoate (1).
2. Results and Discussion
The cyanobacterium Okeania hirsuta, identified using 16S rRNA sequence analysis, was collected from Kuba Beach, Okinawa, Japan, in 2010. A frozen sample of O. hirsuta was extracted with methanol (MeOH). The compound (1) was purified using reversed-phase column chromatography. From 9.7 kg of algal cells, 1.1 mg of 1 was isolated.
Compound (1) was isolated as a colorless amorphous material with no UV absorption above 210 nm. The molecular formula of 1 was C20H36O4S (m/z 373.2408 [M + H]+, calcd. for C20H37O4S m/z 373.2407) via high-resolution electron spray ionization mass spectrometry (HR-ESI-MS), suggesting the presence of a sulfur atom in the molecule. 1H, 13C NMR, and HSQC spectra revealed the presence of a triplet methyl group (δH 0.97) and two singlet methyls (δH 2.79 and 2.80), nine aliphatic methylenes (δC 39.0, 27.6, 30.5, 30.4, 30.6, 26.3, 38.1, 34.4, 21.9), three methines (δH 4.23, 4.12, 4.16), four olefin signals (δC 149.9, 115.7, 123.7, 136.3), and a carbonyl (δC 180.1) (Table 1). Considered from MS and NMR analysis, the unsaturation of 1 was three, which accounted for a carbonyl and two double bonds. Thus, 1 was a C18:2 fatty acid derivative that contained a sulfonium group, two singlet methyls bonding a hetero atom, two hydroxy groups, and a carboxylate in the molecule. Similar to malyngic acid [5] and okeanic acid–A [10], 1 was deduced to be an ω-3 fatty acid derivative by 1H-1H COSY correlation, from H3-18 (δH 0.97) to H-15 (δH 5.31). Another proton connectivity around the hydroxy groups and the double bonds H-15–H2-14–H-13–H2-12–H-11–H-10–H-9–H2-8 was also determined based on the detailed analyses of 1H-1H COSY and TOCSY. The rest of the six methylenes were assigned into a linear alkyl chain from H2-2 to H2-7. An HMBC correlation from H2-2 (δH 2.14) to C-1 (δC 180.1) was observed. Therefore, the skeletal structure of 1 was elucidated. The geometry of Δ10 was deduced to be E from 3JH-10,H-11 (15.5 Hz), and the 13C chemical shift of C-17 at δC 21.7 suggested that the configuration of C-15=C-16 was Z [10]. Two singlet methyls observed at δH 2.80 and 2.79 and δC 23.1 and 23.9, respectively, indicated a dimethylsulfonium group in the molecule [11,12]. The 13C chemical shift (δC 64.7) at C-12 and the HMBC correlation from the singlet methyls to C-12 indicated that the dimethylsulfonium group resided on C-12 (Figure 2). The presence of two hydroxy groups was deduced from the molecular formula obtained from HR-MS results and the 1H and 13C NMR chemical shifts of CH-9 (δH 4.23, δC 72.4) and CH-13 (δH 4.16, δC 72.0). The position of the hydroxy groups was deduced to be at C-9 (δC 72.4) and C-13 (δC 72.0). These MS and NMR analyses allowed us to determine that the structure of okeanoate (1) was (10E,15Z)-12-(dimethylsulfonio)-9,13-dihydroxyoctadeca-10,15-dienoate. Okeanoate (1) was unstable in methanol at 5 °C under dark condition. Within a week, more than half of the isolated 1 changed to other unknown compounds under these conditions. Therefore, biological activity tests could not be performed for 1.

Table 1.
1H (600 MHz) and 13C (150 MHz) NMR data of compound 1 in methanol-d4.
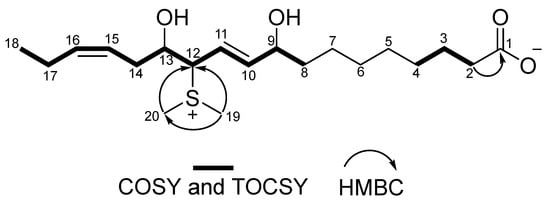
Figure 2.
Key COSY and TOCSY (bold line) and HMBC (arrow, H to C) correlations for okeanoate (1).
3. Material and Methods
3.1. Collection of Cyanobacteria
The cyanobacterium Okeania hirsuta was collected at Kuba Beach, Nakagusuku, in Okinawa Prefecture, Japan, in 2010. O. hirsuta was the dominant cyanobacterial species in all samples. The identification of O. hirsuta was performed based on 16S rRNA sequence analysis [13]. A voucher specimen (20100713-a) was deposited at the Tokyo University of Marine Science and Technology.
3.2. Isolation and Structural Characterization of 1
The frozen O. hirsuta sample (wet weight: 9.7 kg) was extracted with MeOH thrice. After the elimination of MeOH under reduced pressure, the extracts were partitioned between ethyl acetate and H2O. The H2O layer was extracted with 1-butanol (BuOH). After the evaporation of 1-BuOH, the extract was fractionated by MPLC, using ODS gel (Cosmosil 75C18-OPN, Nacalai Tesque Inc., Kyoto, Japan) with stepwise elution with 50%, 70%, 90%, and 100% MeOH [size of the column, 20 × 300 mm; each eluant volume, 200 mL]. The 70% MeOH eluate was subjected to an ODS column (Cosmosil 5C18-AR-II, 10 × 250 mm, Nacalai Tesque Inc., Kyoto, Japan) with 70% MeOH for 10 min, then a gradient elution from 70% to 100% MeOH for 20 min [flow rate, 3.0 mL/min; detection at 210 nm]. The fraction eluted from 5 to 8 min was combined. Final purification was conducted via HPLC using the reversed-phase column (Cosmosil 5C18-AR-II, 4.6 × 250 mm, Nacalai Tesque Inc., Kyoto, Japan) with gradient elution from 45% to 50% MeOH for 0–5 min, then 50% MeOH for 5–15 min [flow rate, 1.0 mL/min; detection at 210 nm]. Okeanoate (1, 1.1 mg) was isolated at a retention time of 7.0 min.
HR ESI-MS was measured with an micrOTOF-QII (Bruker, MA, USA) Quadrupole time-of-flight mass spectrometer (QTOFMS) with high-performance liquid chromatography of the UltiMate 3000 Series (Thermo Fisher Scientific, Billerica, MA, USA). NMR spectra were measured in methanol-d4 at 300 K using a Bruker AVANCE III 600 spectrometer (Bruker Biospin AG, Fällanden, Switzerland).
4. Conclusions
Okeanoate (1), a novel oxylipin, was isolated from the cyanobacterium Okeania hirsuta collected in Okinawa, Japan. In this study, the stereochemistry of the two double bonds could be determined, but only the planar structure could be determined for the other three chiral carbon atoms. To the best of our knowledge, okeanoate (1) is the first oxylipin with a dimethylsulfonium moiety in the middle of the hydrocarbon chain from nature. The biosynthetic pathway to this chemically unique compound is of interest.
Supplementary Materials
Figure S1. 1H-NMR (600 MHz, methanol-d4) spectrum of okeanoate (1). Figure S2. 13C-NMR (150 MHz, methanol-d4) spectrum of okeanoate (1). Figure S3. HSQC spectrum (600 MHz, methanol-d4) of okeanoate (1). Figure S4. HMBC spectrum (600 MHz, methanol-d4) of okeanoate (1). Figure S5. 1H-1H COSY spectrum (600 MHz, methanol-d4) of okeanoate (1). Figure S6. 1H-1H TOCSY spectrum (600 MHz, methanol-d4) of okeanoate (1). Figure S7. HR-ESI-MS spectrum of okeanoate (1). Figure S8. The spectral area between 4.00 and 4.30 ppm of 1H-NMR (600 MHz, methanol-d4) spectrum of okeanoate (1). Figure S9. The spectral area between 5.20 and 6.25 ppm of 1H-NMR (600 MHz, methanol-d4) spectrum of okeanoate (1).
Author Contributions
Conceptualization, H.N. (Hiroshi Nagai) and M.S.; isolation, H.N. (Haruka Nishino); structure analysis, H.N. (Haruka Nishino), B.-T.Z., H.N. (Hiroshi Nagai), and M.S.; analysis, H.U.; writing—original draft preparation, M.S., H.N. (Hiroshi Nagai), H.N. (Haruka Nishino); writing—review and editing, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Japan Society for the Promotion of Science: 22K05817 (Hiroshi Nagai); Japan Society for the Promotion of Science: 23H01962 (M.S.); Japan Science and Technology Agency: JPMJSP2147 (B.Z.).
Data Availability Statement
The spectroscopic data presented in this study are available as Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wasternack, C.; Feussner, I. The oxylipin pathways: Biochemistry and function. Annu. Rev. Plant Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Schilmiller, A.L. Oxylipin metabolism in response to stress. Curr. Opin. Plant Biol. 2002, 5, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Nunnery, J.K.; Mevers, E.; Gerwick, W.H. Biologically active secondary metabolites from marine cyanobacteria. Curr. Opin. Biotechnol. 2010, 21, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.A.; Akhter, N.; Auckloo, B.N.; Khan, I.; Lu, Y.; Wang, K.; Wu, B.; Guo, Y.W. Structural diversity, biological properties and applications of natural products from cyanobacteria. A review. Mar. Drugs 2017, 15, 354. [Google Scholar] [CrossRef] [PubMed]
- Cardellina, J.H.; Moore, R.E. Malyngic acid, a new fatty acid from Lyngbya majuscula. Tetrahedron 1980, 36, 993–996. [Google Scholar] [CrossRef]
- Herz, W.; Kulanthaivel, P. Trihydroxy-C18-acids and a labdane from Rudbeckia fulgida. Phytochemistry 1985, 24, 89–91. [Google Scholar] [CrossRef]
- Lang, I.; Feussner, I. Oxylipin formation in Nostoc punctiforme (PCC73102). Phytochemistry 2007, 68, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Oku, N.; Matsumoto, M.; Yonejima, K.; Tansei, K.; Igarashi, Y. Sacrolide A, a new antimicrobial and cytotoxic oxylipin macrolide from the edible cyanobacterium Aphanothece sacrum. Beilstein J. Org. Chem. 2014, 10, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Oku, N.; Hana, S.; Matsumoto, M.; Yonejima, K.; Tansei, K.; Isogai, Y.; Igarashi, Y. Two new sacrolide-class oxylipins from the edible cyanobacterium Aphanothece sacrum. J. Antibiot. 2017, 70, 708–709. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishino, H.; Kanda, N.; Zhang, B.T.; Kamio, M.; Uchida, H.; Sugahara, K.; Nagai, H.; Satake, M. Okeanic acid–A, a trihydroxy fatty acid from the Okinawan cyanobacterium Okeania hirsuta. Nat. Prod. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, S.; Piattelli, M.; Chillemi, R. (–)-(S)-Dimethylsulfonio-2-methoxybutylate from the red algae Rytiphloea tinctoria. Phytochemistry 1982, 21, 227–228. [Google Scholar] [CrossRef]
- Nakamura, H.; Fujimaki, K.; Sampei, O.; Murai, A. Gonyol: Methionine-induced sulfonium accumulation in a dinoflagellate Gonyaulax polyedra. Tetrahedron Lett. 1993, 34, 8481–8484. [Google Scholar] [CrossRef]
- Kanda, N.; Zhang, B.T.; Shinjo, A.; Kamiya, M.; Nagai, H.; Uchida, H.; Araki, Y.; Nishikawa, T.; Satake, M. 7-Epi-30-Methyloscillatoxin D from an Okinawan cyanobacterium Okeania hirsuta. Nat. Prod. Commun. 2023, 18, 1–5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).