Abstract
The title complex [{PhS(tBuN)2}(Cl)Ge:→RhCl(cod)] (2) was synthesized by the reaction of three-coordinated chlorogermylene, [PhS(tBuN)2]GeCl (1), supported by a diimidosulfinate ligand with a half equivalent of [RhCl(cod)]2 in benzene. The molecular structure of 2 was determined by 1H and 13C NMR spectroscopies and single-crystal X-ray diffraction (SCXRD) analysis. The electronic property of germylene 1 was assessed by determining the Tolman electronic parameter of the corresponding cis-dicarbonyl Rh(I) complex, [{PhS(tBuN)2}(Cl)Ge:→RhCl(CO)2] (3), that was prepared by the treatment of 2 with carbon monoxide.
1. Introduction
In recent years, the utilization of more substantial carbene congeners, commonly known as tetrylenes, as ligands in transition metal chemistry has experienced significant growth [1,2,3,4,5,6,7,8,9,10]. This heightened interest has been propelled by the amphiphilic property of tetrylenes, enabling them to function as both Lewis base and acid, as well as their enhanced electron-donating capacity, surpassing that of most phosphines and N-heterocyclic carbenes (NHCs) [11,12,13]. Indeed, certain tetrylene-transition metal complexes have demonstrated their efficacy as catalysts for homogeneous transformations [14,15,16,17,18,19,20,21,22,23,24]. Furthermore, theoretical calculations support that germylenes can potentially serve as an ancillary ligand for transition metal catalysts compared to common NHC and phosphine ligands [25].
We have been conducting research on the synthesis and properties of a series of three-coordinated tetrylenes supported by iminophsphonamide [Ph2P(RN)2]− (R = tBu, 2,6-iPr2C6H3) [26,27,28,29,30,31,32] and diimidosulfinate [PhS(RN)2]− (R = tBu, SiMe3) [33] ligands, which are isoelectronic ligands of amidinate [R’C(RN)2]−. In particular, we demonstrated that iminophosphonamido silylenes and germylenes not only display strong electron-donating properties surpassing those of NHCs but also exhibit diverse reactivities, including unique coordination behavior [32]. In our ongoing investigation into three-coordinated tetrylenes, we present the synthesis and structure of the germylene-Rh(I) complex [{PhS(tBuN)2}(Cl)Ge:→RhCl(cod)] (cod = 1,5-cyclooctadiene), in which the germylene fragment is supported by a diimidosulfinate ligand, as well as its reactivity toward carbon monoxide.
2. Results and Discussion
The treatment of [PhS(tBuN)2]GeCl 1 [33] with a half equivalent of [RhCl(cod)]2 in benzene gave the corresponding germylene-Rh(I) complex [{PhS(tBuN)2}(Cl)Ge: →RhCl(cod)] 2 as an orange powder in a 66% yield (Scheme 1). In the 1H NMR spectrum of 2, a singlet signal due to tert-butyl groups was observed at 1.34 ppm. Four non-equivalent signals assigned to methylene protons in the cod ligand were found at 1.70–1.78, 2.12, and 2.24 ppm. Two alkenyl protons in the cod ligand appeared at 4.23 and 5.62 ppm as a broad signal. Single crystals of 2 suitable for X-ray diffraction analysis were obtained from a saturated benzene solution at room temperature. The ORTEP of 2 is illustrated in Figure 1, and selected bond lengths and bond angles are provided in Table 1. Complex 2 crystallizes in the triclinic space group P-1 with a benzene molecule per unit cell. As depicted in Figure 1, the central rhodium atom, to which the germanium atom is coordinated, and the phenyl group on the sulfur atom are oriented in opposite directions relative to the four-membered ring GeN2S skeleton. The rhodium atom exhibits a distorted planar square geometry. The Ge–Rh bond length [2.3924(4) Å] of 2, which lies within the range of previously reported germylene-Rh(I) complexes [2.3366(9)–2.4499(8) Å] [34,35,36,37,38,39]. The Ge–Cl bond length [2.2620(6) Å] of 2 is somewhat shorter than that of the starting 1 [2.3498(19) Å] [33]. This shortening of the bond length can be attributed to the coordination of the lone-pair electron on the germanium atom to the rhodium atom, leading to a decrease in electron density on the germanium atom that is a factor to the elongation of the Ge–Cl bond in 1 due to the electronic repulsive interaction between both atoms. The average Rh–C distance (2.204 Å) to the carbon atom (C19, C20) positioned trans to the germanium atom is longer than the distance to the cis-oriented carbon atoms (2.126 Å), indicating the strong trans influence of the germylene ligand.

Scheme 1.
Synthesis of [{PhS(tBuN)2}(Cl)Ge:→RhCl(cod)] 2.
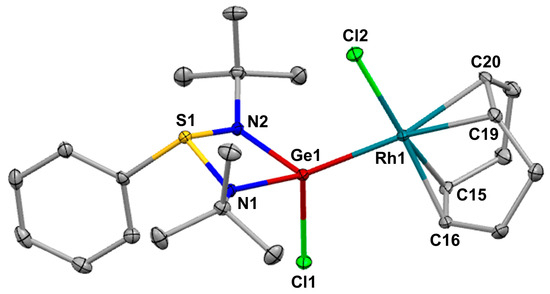
Figure 1.
ORTEP of 2 with thermal ellipsoids at 50% probability. All hydrogen atoms and a molecule of benzene in the unit cell are omitted for clarity.

Table 1.
Selected bond lengths [Å] and bond angles [°].
To assess the electron-donating property of germylene ligand 1 using Tolman’s electronic parameter [40,41], we next conducted the synthesis of the corresponding cis-dicarbonyl Rh(I) complex, [{PhS(tBuN)2}(Cl)Ge:→RhCl(CO)2] (3). Treatment of 2 with carbon monoxide (1 atm) in C6D6 resulted in the quantitative formation of 3 (Scheme 2). In the 13C{1H} NMR spectrum of 3, the carbonyl carbons resonated at 185.8 ppm as a broad signal, which is comparable to that of the germylene-Rh(I) complex bearing an iminophosphonamide ligand, [{Ph2P(tBuN)2}(Cl)Ge:→RhCl(CO)2] (183.1 ppm) [32]. In the IR spectrum of 3, two absorptions due to carbonyl stretching vibration were observed at 2005 and 2069 cm−1. Comparing the average carbonyl stretching frequency (2037 cm−1) with that of the corresponding rhodium complexes bearing common N-heterocyclic carbenes (NHCs) or cyclic (alkyl)(amino) carbenes (cAACs), the donor intensity of germylene 1 is relatively high, falling between NHCs (2046–2051 cm−1) and cAACs (2031–2036 cm−1).
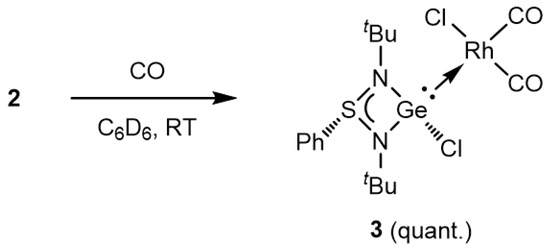
Scheme 2.
Synthesis of [{PhS(tBuN)2}(Cl)Ge:→RhCl(CO)2] 3.
3. Materials and Methods
3.1. General Considerations
Unless otherwise noted, all experiments were carried out under an argon atmosphere using standard Schlenk-line techniques or a glovebox (UNICO Ltd., Ibaraki, Japan). 1H and 13C NMR spectra were recorded on Bruker Avance-500 (500 MHz for 1H) and Bruker Avance-400 (101 MHz for 13C) spectrometers (Bruker, Kanagawa, Japan) using C6D6 as the solvent at room temperature. IR spectrum was recorded on a TENSOR II (Bruker, Kanagawa, Japan). All melting points were determined on a Mel-Temp capillary tube apparatus and were uncorrected. Elemental analyses were conducted at the Molecular Analysis and Life Science Center of Saitama University. All solvents were dried over 4A molecular sieves or potassium mirrors before use. All materials were obtained from commercial suppliers and used without further purification except 1 that was prepared according to the corresponding literature procedure [33].
3.2. Synthesis of [{PhS(tBuN)2}(Cl)Ge:→RhCl(cod)] 2
In a Schlenck tube, germylene 1 (52.1 mg, 0.14 mmol) and [Rh(cod)Cl]2 (35.8 mg, 0.07 mmol) were dissolved into benzene (1 mL). The resulting orange solution was stirred for 4 h at room temperature. All volatiles were removed under reduced pressure to give germylene-rhodium(I) complex 2 (57.8 mg, 66%) as orange crystals. Mp. 165–167 °C (decomp.). 1H NMR (C6D6, 500 MHz): δ = 1.34 (s, 18H, CH3), 1.71–1.78 (m, 4H, CH2), 2.12 (br s, 2H, CH2), 2.25 (br s, 2H, CH2), 4.43 (br s, 2H, CH), 5.62 (br s, 2H, CH), 6.93–6.95 (m, 3H, Ph), 8.03–8.05 (m, 2H, Ph). 13C{1H} NMR (C6D6, 101 MHz): δ 29.3 (CH2), 31.0 (CH3), 33.5 (CH2), 56.1 (C), 70.2 (CH, JRh-C = 14 Hz, cod), 101.0 (CH, cod), 128.6 (CH, Ph), 129.9 (CH, Ph), 133.8 (CH, Ph), 147.3 (C, Ph). Anal. Calcd. for C22H35Cl2GeN2RhS: C, 43.60; H, 5.82; N, 4.62. Found: C, 43.98; H, 5.71; N, 4.46. See Supplementary Materials.
3.3. Reaction of 2 with CO
A pressure-tight NMR tube containing a solution of 2 in C6D6 (0.5 mL) under a CO pressure (ca. 3 atm) was kept for 1 h at room temperature. The color of the solution changed immediately from orange to yellow. The reaction was completed within 1 h. 1H NMR (C6D6, 500 MHz): δ 1.28 (s, 9H, CH3), 1.58 (s, 9H, CH3), 6.95 (t, 1H, 3J = 7 Hz, p-Ph), 7.06 (t, 2H, 3J = 8 Hz, m-Ph), 7.51 (d, 2H, 3J = 8 Hz, o-Ph). 13C{1H} NMR (C6D6, 101 MHz): δ 33.0 (CH3), 33.6 (CH3), 60.0 (C), 62.4 (C), 128.6 (CH, Ph), 129.2 (CH, Ph), 131.2 (CH, Ph), 145.6 (C, Ph), 185.8 (C, CO). IR (C6D6): 2005, 2069 cm−1 [ν(CO)]. See Supplementary Materials. Concentration of the reaction mixture resulted in the decomposition of the expected product 3.
3.4. SCXRD Analysis of 2
An orange single crystal of 2 was grown from a saturated benzene solution at 25 °C. The intensity data were collected at 100 K on a Bruker SMART APEX II diffractometer employing graphite-monochromated MoKα radiation (λ = 0.71073 Å). The structure was solved by direct methods (SHELXT) [42] and refined by full-matrix least-squares procedures on F2 for all reflections (SHELXL) [43]. Hydrogen atoms were located by assuming ideal geometry and were included in the structure calculations without further refinement of the parameters.
Crystal data for C28H41Cl2GeN2RhS (2): M = 684.09 g mol−1, triclinic, P-1, a = 8.3881(7), b = 12.5836(11), c = 14.1437(12) Å, α = 88.7760(10), β = 88.8650(10), γ = 89.9720(10)°, V = 1492.3(2) Å3, Z = 2, Dx = 1.522 g cm−3, F(000) = 700, and μ = 1.829 mm−1. CCDC deposition number: 2324776.
4. Conclusions
A novel germylene-rhodium(I) complex 2 was synthesized and structurally characterized by spectroscopic data and SCXRD. To estimate the electron-donating ability of diimidosulfinato germylene ligand 1, the corresponding cis-dicarbonyl Rh(I) complex 3 was prepared through the reaction of 2 with carbon monoxide. The donor property of 1 was determined from the Tolman electronic parameter of 3 and revealed to be intermediate between NHCs and cAACs.
Supplementary Materials
The following are available online: all spectroscopic data for 2 and 3 and crystallographic data for 2 in Crystallographic Information File (CIF) format. CCDC 2324776 also contains the supplementary crystallographic data for this paper.
Author Contributions
Conceptualization, N.H. and N.N.; methodology, N.N.; formal analysis, N.H.; investigation, N.H.; resources, N.N.; data curation, N.N.; writing—original draft preparation, N.H.; writing—review and editing, A.I. and N.N.; visualization, N.N.; supervision, N.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by JSPS KAKENHI (grant number: JP22K05138 to N.N.).
Data Availability Statement
CCDC 2324776 (2) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif (accessed on 11 January 2024), or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kühl, O. N-heterocyclic germylenes and related compounds. Coord. Chem. Rev. 2004, 248, 411–427. [Google Scholar] [CrossRef]
- Waterman, R.; Hayes, P.G.; Tilley, T.D. Synthetic Development and Chemical Reactivity of Transition-Metal Silylene Complexes. Acc. Chem. Res. 2007, 40, 712–719. [Google Scholar] [CrossRef]
- Nagendran, S.; Roesky, H.W. The chemistry of aluminum(I), silicon(II), and germanium(II). Organometallics 2008, 27, 457–492. [Google Scholar] [CrossRef]
- Mizuhata, Y.; Sasamori, T.; Tokitoh, N. Stable heavier carbene analogues. Chem. Rev. 2009, 109, 3479–3511. [Google Scholar] [CrossRef]
- Blom, B.; Stoelzel, M.; Driess, M. New vistas in N-heterocyclic silylene (NHSi) transition-metal coordination chemistry: Syntheses, structures and reactivity towards activation of small molecules. Chem. Eur. J. 2013, 19, 40–62. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rodríguez, L.; Cabeza, J.A.; García-Álvarez, P.; Polo, D. The transition-metal chemistry of amidinatosilylenes, -germylenes and -stannylenes. Coord. Chem. Rev. 2015, 300, 1–28. [Google Scholar] [CrossRef]
- Cabeza, J.A.; García-Álvarez, P.; Polo, D. Intramolecularly Stabilized Heavier Tetrylenes: From Monodentate to Bidentate Ligands. Eur. J. Inorg. Chem. 2016, 2016, 10–22. [Google Scholar] [CrossRef]
- Krahfuss, M.J.; Radius, U. N-Heterocyclic silylenes as ambiphilic activators and ligands. Dalton Trans. 2021, 50, 6752–6765. [Google Scholar] [CrossRef]
- Cabeza, J.A.; García-Álvarez, P. Cyclometallation of Heavier Tetrylenes: Reported Complexes and Applications in Catalysis. Eur. J. Inorg. Chem. 2021, 2021, 3315–3326. [Google Scholar] [CrossRef]
- Komuro, T.; Nakajima, Y.; Takaya, J.; Hashimoto, H. Recent progress in transition metal complexes supported by multidentate ligands featuring group 13 and 14 elements as coordinating atoms. Coord. Chem. Rev. 2022, 473, 214837. [Google Scholar] [CrossRef]
- Cabeza, J.A.; García-Álvarez, P.; Pérez-Carreño, E.; Polo, D. Ring Opening and Bidentate Coordination of Amidinate Germylenes and Silylenes on Carbonyl Dicobalt Complexes: The Importance of a Slight Difference in Ligand Volume. Chem.—Eur. J. 2014, 20, 8654–8663. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodriguez, L.; Cabeza, J.A.; García-Álvarez, P.; Pérez-Carreño, E.; Polo, D. Amidinatogermylene Derivatives of Ruthenium Carbonyl: New Insights into the Reactivity of [Ru3(CO)12] with Two-Electron-Donor Reagents of High Basicity. Inorg. Chem. 2015, 54, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Benedek, Z.; Szilvási, T. Can low-valent silicon compounds be better transition metal ligands than phosphines and NHCs? RSC Adv. 2015, 5, 5077–5086. [Google Scholar] [CrossRef]
- Calimano, E.; Tilley, T.D. Alkene Hydrosilation by a Cationic Hydrogen-Substituted Iridium Silylene Complex. J. Am. Chem. Soc. 2008, 130, 9226–9227. [Google Scholar] [CrossRef] [PubMed]
- Calimano, E.; Tilley, T.D. Synthesis and Structure of PNP-Supported Iridium Silyl and Silylene Complexes: Catalytic Hydrosilation of Alkenes. J. Am. Chem. Soc. 2009, 131, 11161–11173. [Google Scholar] [CrossRef] [PubMed]
- Fasulo, M.E.; Lipke, M.C.; Tilley, T.D. Structural and mechanistic investigation of a cationic hydrogen-substituted ruthenium silylene catalyst for alkene hydrosilation. Chem. Sci. 2013, 4, 3882–3887. [Google Scholar] [CrossRef]
- Kireenko, M.M.; Zaitsev, K.V.; Oprunenko, Y.F.; Churakov, A.V.; Tafeenko, V.A.; Karlov, S.S.; Zaitseva, G.S. Palladium complexes with stabilized germylene and stannylene ligands. Dalton Trans. 2013, 42, 7901–7912. [Google Scholar] [CrossRef]
- Blom, B.; Gallego, D.; Driess, M. N-heterocyclic silylene complexes in catalysis: New frontiers in an emerging field. Inorg. Chem. Front. 2014, 1, 134–148. [Google Scholar] [CrossRef]
- Smart, K.A.; Mothes-Martin, E.; Vendier, L.; Perutz, R.N.; Grellier, M.; Sabo-Etienne, S. A Ruthenium Dihydrogen Germylene Complex and the Catalytic Synthesis of Digermoxane. Organometallics 2015, 34, 4158–4163. [Google Scholar] [CrossRef]
- Zhou, Y.-P.; Raoufmoghaddam, S.; Szilvási, T.; Driess, M. A Bis(silylene)-Substituted ortho-Carborane as a Superior Ligand in the Nickel-Catalyzed Amination of Arenes. Angew. Chem. Int. Ed. 2016, 55, 12868–12872. [Google Scholar] [CrossRef]
- Iimura, T.; Akasaka, N.; Iwamoto, T. A Dialkylsilylene-Pt(0) Complex with a DVTMS Ligand for the Catalytic Hydrosilylation of Functional Olefins. Organometallics 2016, 35, 4071–4076. [Google Scholar] [CrossRef]
- Iimura, T.; Akasaka, N.; Kosai, T.; Iwamoto, T. A Pt(0) complex with cyclic (alkyl)(amino)silylene and 1,3-divinyl-1,1,3,3-tetramethyldisiloxane ligands: Synthesis, molecular structure, and catalytic hydrosilylation activity. Dalton Trans. 2017, 46, 8868–8874. [Google Scholar] [CrossRef]
- Schmidt, M.; Blom, B.; Szilvási, T.; Schomacker, R.; Driess, M. Improving the Catalytic Activity in the Rhodium-Mediated Hydroformylation of Styrene by a Bis(N-heterocyclic silylene) Ligand. Eur. J. Inorg. Chem. 2017, 2017, 1284–1291. [Google Scholar] [CrossRef]
- Zhou, Y.-P.; Driess, M. Isolable Silylene Ligands Can Boost Efficiencies and Selectivities in Metal-Mediated Catalysis. Angew. Chem. Int. Ed. 2019, 58, 3715–3728. [Google Scholar] [CrossRef]
- Benedek, Z.; Szilvási, T. Theoretical Assessment of Low-Valent Germanium Compounds as Transition Metal Ligands: Can They Be Better than Phosphines or NHCs? Organometallics 2017, 36, 1591–1600. [Google Scholar] [CrossRef]
- Takahashi, S.; Sekiguchi, J.; Ishii, A.; Nakata, N. An iminophosphonamido-chlorosilylene as a strong σ-donating NHSi ligand: Synthesis and coordination chemistry. Angew. Chem. Int. Ed. 2021, 133, 4101–4105. [Google Scholar] [CrossRef]
- Takahashi, S.; Ishii, A.; Nakata, N. Interconversion between a silaimine and an aminosilylene supported by an iminophosphonamide ligand. Chem. Commun. 2021, 57, 3203–3206. [Google Scholar] [CrossRef]
- Takahashi, S.; Ishii, A.; Nakata, N. Formation of silaimines from a sterically demanding iminophosphonamido chlorosilylene via intramolecular N–P bond cleavage. Chem. Commun. 2021, 57, 6728–6731. [Google Scholar] [CrossRef]
- Nakaya, K.; Takahashi, S.; Ishii, A.; Boonpalit, K.; Surawatanawong, P.; Nakata, N. Hydroboration of carbonyls and imines by an iminophosphonamido Tin(II) precatalyst. Dalton Trans. 2021, 50, 14810–14819. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Sekiguchi, J.; Nakaya, K.; Ishii, A.; Nakata, N. Halogen-exchange reactions of iminophosphonamido-chlorosilylenes with alkali halides: Convenient synthesis of heavier halosilylenes. Inorg. Chem. 2022, 61, 7266–7273. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakaya, K.; Ishii, A.; Nakata, N. [N,N′-Di-tert-butyl-P,P-diphenylphosphinimidic Amidato-κN,κN′]chlorosilicon-κSi-tetracarbonyliron. Molbank 2022, 2022, M1433. [Google Scholar] [CrossRef]
- Takahashi, S.; Kamiyama, S.; Ishii, A.; Nakata, N. Syntheses of Iminophosphomamido Chlorogermylenes and Their Complexation with a Rhodium(I) Complex. Chem. Asian J. 2023, 2023, e202300968. [Google Scholar] [CrossRef]
- Nakata, N.; Hosoda, N.; Takahashi, S.; Ishii, A. Chlorogermylenes and -stannylenes stabilized by diimidosulfinate ligands: Synthesis, structures, and reactivity. Dalton Trans. 2018, 47, 481–490. [Google Scholar] [CrossRef]
- Veith, M.; Müller, A.; Stahl, L.; Nötzel, M.; Jarczyk, M.; Huch, V. Formation of Metal Clusters or Nitrogen-Bridged Adducts by Reaction of a Bis(amino)stannylene with Halides of Two-Valent Transition Metals. Inorg. Chem. 1996, 35, 3848–3855. [Google Scholar] [CrossRef]
- García, J.M.; Ocando-Mavárez, E.; Kato, T.; Coll, D.S.; Briceño, A.; Saffon-Merceron, N.; Baceiredo, A. Synthesis and Characterization of Rhodium Complexes with Phosphine-Stabilized Germylenes. Inorg. Chem. 2012, 51, 8187–8193. [Google Scholar] [CrossRef]
- Matioszek, D.; Saffon, N.; Sotiropoulos, J.-M.; Miqueu, K.; Castel, A.; Escudié, J. Bis(amidinato)germylenerhodium Complexes: Synthesis, Structure, and Density Functional Theory Calculations. Inorg. Chem. 2012, 51, 11716–11721. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, L.; Cabeza, J.A.; Fernández-Colinas, J.M.; García-Álvarez, P.; Polo, D. Amidinatogermylene Metal Complexes as Homogeneous Catalysts in Alcoholic Media. Organometallics 2016, 35, 2516–2523. [Google Scholar] [CrossRef]
- Su, B.; Ota, K.; Kinjo, R. Germylone-bridged bimetallic Ir and Rh complexes. Dalton Trans. 2019, 48, 3555–3559. [Google Scholar] [CrossRef]
- Poitiers, N.E.; Giarrana, L.; Huch, V.; Zimmer, M.; Scheschkewitz, D. Exohedral Functionalization vs. Core Expansion of Siliconoids with Group 9 Metals: Catalytic Activity in Alkene Isomerization. Chem. Sci. 2020, 11, 7782–7788. [Google Scholar] [CrossRef]
- Melaimi, M.; Soleilhavoup, M.; Bertrand, G. Stable Cyclic Carbenes and Related Species beyond Diaminocarbenes. Angew. Chem. Int. Ed. 2010, 49, 8810–8849. [Google Scholar] [CrossRef]
- Martin, D.; Melaimi, M.; Soleilhavoup, M.; Bertrand, G. A Brief Survey of Our Contribution to Stable Carbene Chemistry. Organometallics 2011, 30, 5304–5313. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).