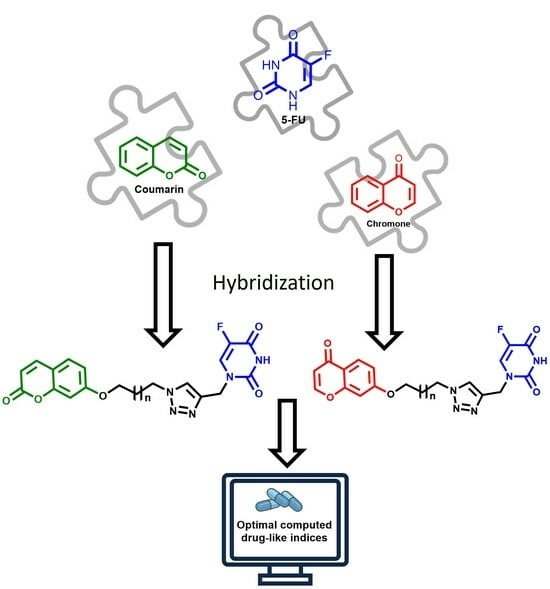
5-Fluorouracil/Coumarin and 5-Fluorouracil/Chromone Hybrids: Synthesis and Drug-Likeness Modeling
Abstract
1. Introduction
2. Results and Discussion
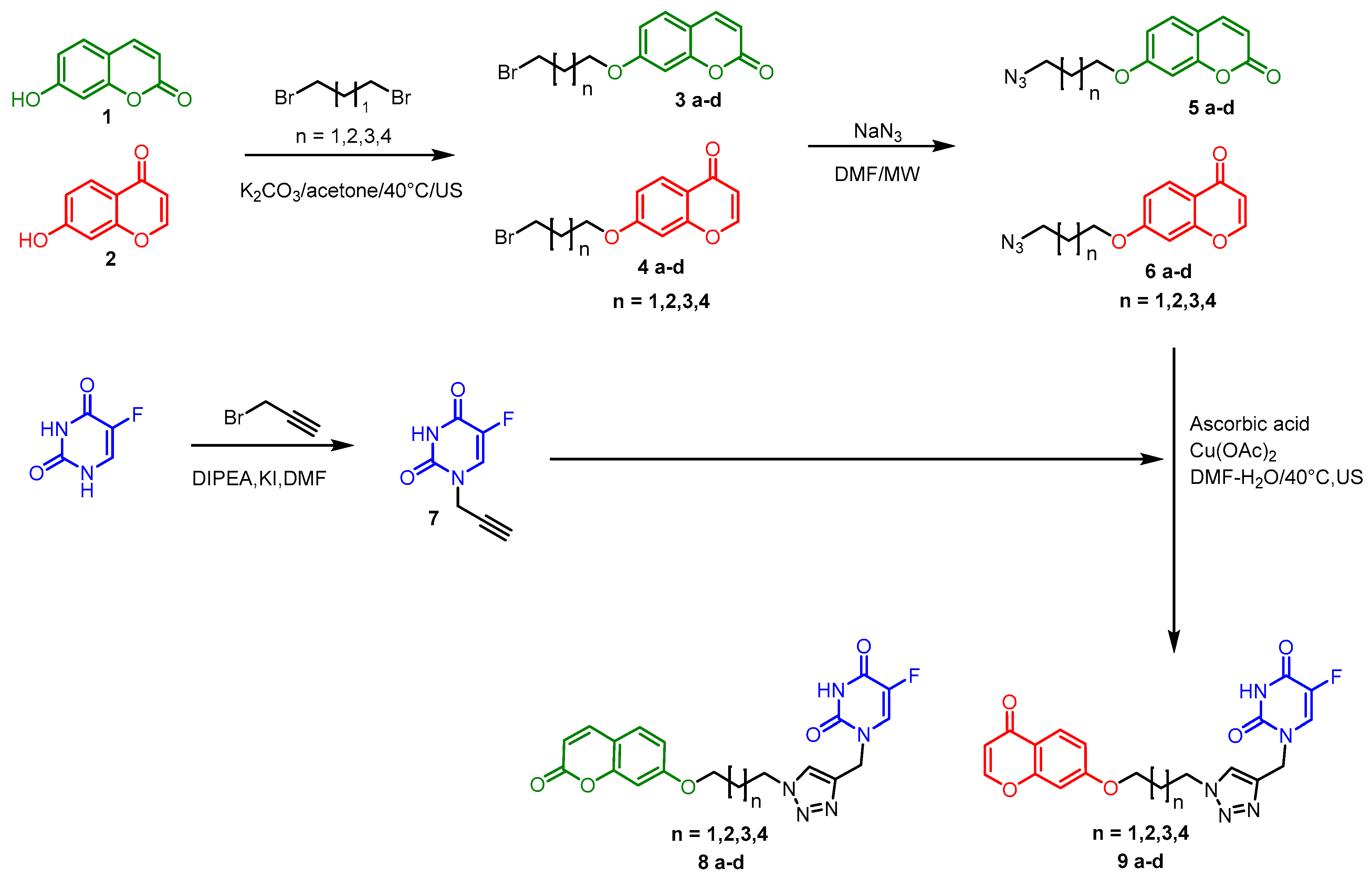
2.1. Chemistry
2.2. Theoretical Pharmacokinetic and Drug-Likeness Studies for Hybrids 8a–d and 9a–d
3. Materials and Methods
3.1. Chemical Synthesis

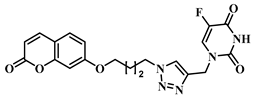
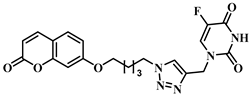
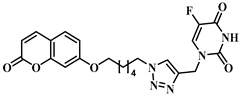

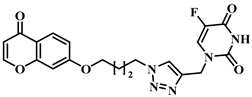
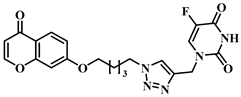
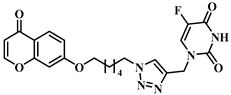
3.2. Theoretical Drug-Likeness Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reis, J.; Gaspar, A.; Milhazes, N.; Borges, F. Chromone as a Privileged Scaffold in Drug Discovery: Recent Advances. J. Med. Chem. 2017, 60, 7941–7957. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, N.U.A.; Irfan, M.; Hassan, S.U.; Saleem, U. Current Strategies in Development of New Chromone Derivatives with Diversified Pharmacological Activities: A Review. Pharm. Chem. J. 2020, 54, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.; Bansal, Y.; Silakari, O.; Bansal, G. Coumarin hybrids as novel therapeutic agents. Bioorg. Med. Chem. 2014, 22, 3806–3814. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.D.; Jiang, Y.Y.; Guo, F.X.; Chen, L.X.; Xu, L.L.; Zhang, W.; Liu, B. The antitumor activity of naturally occurring chromones: A review. Fitoterapia 2019, 135, 114–129. [Google Scholar] [CrossRef]
- Song, X.F.; Fan, J.; Liu, L.; Liu, X.F.; Gao, F. Coumarin derivatives with anticancer activities: An update. Arch. Pharm. 2020, 353, e2000025. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Sabt, A.; Elkaeed, E.B.; Eldehna, W.M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg. Chem. 2020, 103, 104163. [Google Scholar] [CrossRef] [PubMed]
- Banikazemi, Z.; Mirazimi, S.M.; Dashti, F.; Mazandaranian, M.R.; Akbari, M.; Morshedi, K.; Aslanbeigi, F.; Rashidian, A.; Chamanara, M.; Hamblin, M.R.; et al. Coumarins and Gastrointestinal Cancer: A New Therapeutic Option? Front. Oncol. 2021, 11, 752784. [Google Scholar] [CrossRef]
- Herrera, R.A.; Moreno, G.; Araque, P.; Vásquez, I.; Naranjo, E.; Alzate, F.; Cardona, G.W. In-vitro Chemopreventive Potential of a Chromone from Bomarea setacea (ALSTROEMERIACEAE) against Colorectal Cancer. Iran. J. Pharm. Res. 2021, 20, 254. [Google Scholar]
- Liu, W.; Hua, J.; Zhou, J.; Zhang, H.; Zhu, H.; Cheng, Y.; Gust, R. Synthesis and in vitro antitumor activity of novel scopoletin derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5008. [Google Scholar] [CrossRef]
- Kolodziej, H.; Kayser, O.; Woerdenbag, H.J.; van Uden, W.; Pras, N. Structure-cytotoxicity relationships of a series of natural and semi-synthetic simple coumarins as assessed in two human tumour cell lines. Z. Naturforsch. 1997, 52, 240. [Google Scholar] [CrossRef]
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [CrossRef]
- Campos, J.; Domínguez, J.F.; Gallo, M.A.; Espinosa, A. From a classic approach in cancer chemotherapy towards differentiation therapy: Acyclic and cyclic seven-membered 5-fluorouracil O,N-acetals. Curr. Pharm. Des. 2000, 18, 1797–1810. [Google Scholar] [CrossRef]
- Cardona, G.W.; Herrera, R.A.; Castrillón, L.W.; Ramírez-Malule, H. Chemistry and Anticancer Activity of Hybrid Molecules and Derivatives based on 5-Fluorouracil. Curr. Med. Chem. 2021, 28, 5551–5601. [Google Scholar] [CrossRef] [PubMed]
- Keith, C.T.; Borisy, A.; Stockwell, B.R. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 2005, 4, 71–78. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef]
- de Oliveira Pedrosa, M.; Duarte da Cruz, R.M.; de Oliveira Viana, J.; de Moura, R.O.; Ishiki, H.M.; Barbosa Filho, J.M.; Diniz, M.F.; Scotti, M.T.; Scotti, L.; Bezerra Mendonca, F.J. Hybrid Compounds as Direct Multitarget Ligands: A Review. Curr. Top. Med. Chem. 2017, 17, 1044–1079. [Google Scholar] [CrossRef]
- Cardona, G.W.; Yepes, A.F.; Herrera, R.A. Hybrid Molecules: Promising Compounds for the Development of New Treatments Against Leishmaniasis and Chagas Disease. Curr. Med. Chem. 2018, 25, 3637–3679. [Google Scholar] [CrossRef]
- Kaur, J.; Saxena, M.; Rishi, N. An Overview of Recent Advances in Biomedical Applications of click chemistry. Bioconjug. Chem. 2021, 32, 1455–1471. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y. Application of “Click” Chemistry in Biomedical Hydrogels. ACS Omega 2022, 7, 36918–36928. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar Yadav, A.; Mishra, V.; Kumar, D. Recent Advancements in Triazole-based click chemistry in Cancer Drug Discovery and Development. SynOpen 2023, 7, 186–208. [Google Scholar]
- Moreno-Quintero, G.; Betancur-Zapata, E.; Herrera-Ramírez, A.; Cardona-Galeano, W. New Hybrid Scaffolds Based on 5-FU/Curcumin: Synthesis, Cytotoxic, Antiproliferative and Pro-Apoptotic Effect. Pharmaceutics 2023, 15, 1221. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Quintero, G.; Castrillón-Lopez, W.; Herrera-Ramirez, A.; Yepes-Pérez, A.F.; Quintero-Saumeth, J.; Cardona-Galeano, W. Synthesis and Chemopreventive Potential of 5-FU/Genistein Hybrids on Colorectal Cancer Cells. Pharmaceuticals 2022, 15, 1299. [Google Scholar] [CrossRef] [PubMed]
- Castrillón-López, W.; Yepes-Pérez, A.F.; Cardona-Galeano, W. Synthesis and In Silico Drug-Likeness Modeling of 5-FU/ASA Hybrids. Molbank 2023, 2023, M1745. [Google Scholar] [CrossRef]
- Otero, E.; García, E.; Palacios, G.; Yepes, L.M.; Carda, M.; Agut, R.; Vélez, I.D.; Cardona, W.I.; Robledo, S.M. Triclosan-caffeic acid hybrids: Synthesis, leishmanicidal, trypanocidal and cytotoxic activities. Eur. J. Med. Chem. 2017, 141, 73–83. [Google Scholar] [CrossRef]
- García, E.; Coa, J.C.; Otero, E.; Carda, M.; Vélez, I.D.; Robledo, S.M.; Cardona, W.I. Synthesis and antiprotozoal activity of furanchalcone–quinoline, furanchalcone–chromone and furanchalcone-imidazole hybrids. Med. Chem. Res. 2018, 27, 497–511. [Google Scholar] [CrossRef]
- Gómez-R, L.; Moreno-Q, G.; Herrera-R, A.; Castrillón-L, W.; Yepes, A.F.; Cardona-Galeano, W. New Hybrid Scaffolds Based on ASA/Genistein: Synthesis, Cytotoxic Effect, Molecular Docking, Drug-likeness and in silico ADME/tox Modeling. J. Appl. Pharm. Sci. 2022, 12, 15–30. [Google Scholar]
- Pessel, F.; Billault, I.; Scherrmann, M.C. Total synthesis of triazole-linked C-glycosyl flavonoids in alternative solvents and environmental assessment in terms of reaction, workup and purification. Green Chem. 2016, 18, 5558–5568. [Google Scholar] [CrossRef]
- Halay, E.; Ay, E.; Şalva, E.; Ay, K.; Karayıldırım, T. Syntheses of 1,2,3-triazole-bridged pyranose sugars with purine and pyrimidine nucleobases and evaluation of their anticancer potential. Nucleosides Nucleotides Nucleic Acids 2017, 36, 598–619. [Google Scholar]
- Shinde, V.; Mhaske, P.C.; Singh, A.; Sarkar, D.; Mahulikar, P. Synthesis and biological evaluation of new 4-(4-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl)-2-phenyl thiazole derivatives. J. Heterocycl. Chem. 2019, 56, 3093–3101. [Google Scholar] [CrossRef]
- Ditzinger, F.; Price, D.J.; Ilie, A.R.; Köhl, N.J.; Jankovic, S.; Tsakiridou, G.; Aleandri, S.; Kalantzi, L.; Holm, R.; Nair, A.; et al. Lipophilicity and hydrophobicity considerations in bio-enabling oral formulations approaches—A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 464–482. [Google Scholar] [CrossRef]
- Pham-The, H.; Cabrera-Pérez, M.Á.; Nam, N.H.; Castillo-Garit, J.A.; Rasulev, B.; Le-Thi-Thu, H.; Casañola-Martin, G.M. In Silico Assessment of ADME Properties: Advances in Caco-2 Cell Monolayer Permeability Modeling. Curr. Top. Med. Chem. 2018, 18, 2209–2229. [Google Scholar] [CrossRef]
- Broccatelli, F.; Salphati, L.; Plise, E.; Cheong, J.; Gobbi, A.; Lee, M.L.; Aliagas, I. Predicting Passive Permeability of Drug-like Molecules from Chemical Structure: Where Are We? Mol. Pharm. 2016, 13, 4199–4208. [Google Scholar] [CrossRef]
- Press, B.; Di Grandi, D. Permeability for intestinal absorption: Caco-2 assay and related issues. Curr. Drug Metab. 2008, 9, 893–900. [Google Scholar] [CrossRef]
- Ertl, P.; Rohdem, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Zhivkova, Z.D. Studies on drug-human serum albumin binding: The current state of the matter. Curr. Pharm. Des. 2015, 21, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Colmenarejo, G. In silico prediction of drug-binding strengths to human serum albumin. Med. Res. Rev. 2003, 23, 275–301. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.F.; Hamilton, D.J.; de Esch, I.J.P.; Wijtmans, M.; O’Brien, P. Escape from planarity in fragment-based drug discovery: A synthetic strategy analysis of synthetic 3D fragment libraries. Drug Discov. Today 2022, 27, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Benardout, M.; Le Gresley, A.; ElShaer, A.; Wren, S.P. Application of fSP3 towards Non-Systemic Drug Discovery. 2023; pre-print. [Google Scholar] [CrossRef]
- Wei, W.; Cherukupalli, S.; Jing, L.; Liu, X.; Zhan, P. Fsp3: A new parameter for drug-likeness. Drug Discov. Today 2020, 25, 1839–1845. [Google Scholar] [CrossRef]
- Ward, S.E.; Beswick, P. What does the aromatic ring number mean for drug design? Expert Opin. Drug Discov. 2014, 9, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, T.J.; Macdonald, S.J.; Young, R.J.; Pickett, S.D. The impact of aromatic ring count on compound developability: Further insights by examining carbo- and hetero-aromatic and -aliphatic ring types. Drug Discov. Today 2011, 16, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Bajorath, J. Evolution of assay interference concepts in drug discovery. Expert Opin. Drug Discov. 2021, 16, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Symeonidis, T.; Chamilos, M.; Hadjipavlou-Litina, D.J.; Kallitsakis, M.; Litinas, K.E. Synthesis of hydroxycoumarins and hydroxybenzo[f]- or [h]coumarins as lipid peroxidation inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 1139–1142. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, X.B.; Xie, S.S.; Jiang, N.; Wang, K.D.; Yao, H.Q.; Sun, H.B.; Kong, L.Y. Multifunctional tacrine-flavonoid hybrids with cholinergic, β-amyloid-reducing, and metal chelating properties for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013, 69, 632–646. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
| Properties | Hybrid | |||||||
|---|---|---|---|---|---|---|---|---|
| 8a | 8b | 8c | 8d | 9a | 9b | 9c | 9d | |
| MW a | 413.364 | 427.391 | 441.418 | 455.44 | 413.364 | 427.391 | 441.418 | 455.44 |
| TPSA b | 125.01 | 125.01 | 125.01 | 125.01 | 125.01 | 125.01 | 125.01 | 125.01 |
| n-RB c | 7 | 8 | 9 | 10 | 7 | 8 | 9 | 10 |
| n-ON d | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| n-OHNH e | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| log Po/w f | 1.31 | 1.70 | 2.09 | 2.48 | 1.31 | 1.70 | 2.09 | 2.48 |
| logKHSA g | −0.314 | −0.216 | −0.231 | −0.034 | −0.314 | −0.216 | −0.231 | −0.034 |
| Fsp3 h | 0.21 | 0.25 | 0.29 | 0.32 | 0.21 | 0.25 | 0.29 | 0.32 |
| #ArRNG i | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Caco-2 j | 141 | 111 | 94 | 115 | 141 | 111 | 94 | 115 |
| App. MDCK k | 106 | 81 | 46 | 84 | 106 | 81 | 46 | 84 |
| PAINS l | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraldo-Arroyave, L.; Yepes, A.F.; Cardona-Galeano, W. 5-Fluorouracil/Coumarin and 5-Fluorouracil/Chromone Hybrids: Synthesis and Drug-Likeness Modeling. Molbank 2024, 2024, M1779. https://doi.org/10.3390/M1779
Giraldo-Arroyave L, Yepes AF, Cardona-Galeano W. 5-Fluorouracil/Coumarin and 5-Fluorouracil/Chromone Hybrids: Synthesis and Drug-Likeness Modeling. Molbank. 2024; 2024(1):M1779. https://doi.org/10.3390/M1779
Chicago/Turabian StyleGiraldo-Arroyave, Laura, Andrés F. Yepes, and Wilson Cardona-Galeano. 2024. "5-Fluorouracil/Coumarin and 5-Fluorouracil/Chromone Hybrids: Synthesis and Drug-Likeness Modeling" Molbank 2024, no. 1: M1779. https://doi.org/10.3390/M1779
APA StyleGiraldo-Arroyave, L., Yepes, A. F., & Cardona-Galeano, W. (2024). 5-Fluorouracil/Coumarin and 5-Fluorouracil/Chromone Hybrids: Synthesis and Drug-Likeness Modeling. Molbank, 2024(1), M1779. https://doi.org/10.3390/M1779