Abstract
The reaction of (−)-nopol mesylate with piperazine in acetonitrile under reflux, afforded symmetric 1,4-bis(2-((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl)ethyl)piperazine in a good yield. The compound was fully characterized and its structure was confirmed using X-ray diffraction analysis.
1. Introduction
Natural products such as monoterpenes and their derivatives represent valuable and renewable resources for organic chemistry. Their inherent attributes, encompassing availability, renewability, often high optical purity, and versatile reactivity make them an attractive basis for diverse transformations including the synthesis of novel chiral heterocyclic bioactive compounds [1,2].
In recent endeavors directed towards the synthesis of azole–monoterpene hybrids with antifungal activity, we successfully obtained piperazine derivatives containing various monoterpene fragments, for example 1-(2-((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl)ethyl)piperazine 1 was derived from (−)-nopol mesylate (Scheme 1) [3]. However, during the synthesis, in addition to monosubstituted piperazine 1, the formation of product 2 with an m/z value of 382 Da in GC/MS spectrum, which corresponds to the attachment of two monoterpene moieties to piperazine was also observed, but this minor compound was not isolated due to its insignificant amount in the reaction mixture.
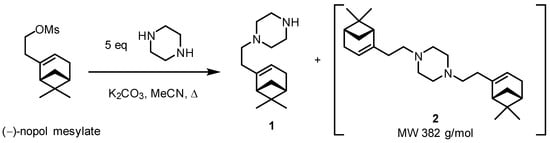
Scheme 1.
Synthesis of (−)-nopol piperazine derivatives [3].
Piperazine has attracted considerable interest in the field of medicinal chemistry owing to its diverse pharmacological effects [4]. Various derivatives of piperazine commonly demonstrate a fascinating spectrum of therapeutic activities, including but not limited to antibacterial, antiviral, antitumor, antifungal, and anticonvulsant properties [5,6].
Moreover, the piperazine moiety is acknowledged as a valuable pharmacophore in combating mycobacterial infections, demonstrating a commendable pharmacological profile [7]. One of the strategies for searching for new anti-tuberculosis drugs is to obtain structural analogues of the well-known antituberculosis drug ethambutol (Figure 1). One of the promising drugs obtained using this approach is a geraniol derivative SQ109 with excellent anti-tuberculosis activity [8]. Bogatcheva et al. [9] showed that a series of monoterpene–piperazine conjugates demonstrated promising anti-tuberculosis activity. Thus, symmetrical monoterpene–piperazine derivatives hold considerable scientific interest for their synthesis and subsequent exploration of their anti-tubercular activity. This is why we decided to obtain compound 2 and elucidate its structure, which was the objective of the current work.
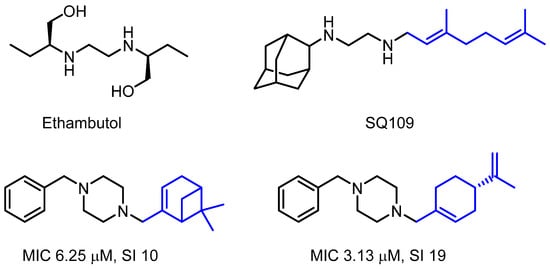
Figure 1.
Anti-tubercular drug ethambutol and monoterpene derivatives with anti-tuberculosis activity. MIC—minimum inhibitory concentration, SI = CC50/MIC—selectivity index.
2. Results and Discussion
For the selective synthesis of 1,4-bis-((−)-nopol)piperazine 2, we performed the reaction of (−)-nopol with methanesulfonyl chloride (MsCl) according to [3] to obtain a corresponding mesylate with 95% yield. (−)-Nopol mesylate then reacted with 0.5 equivalents of piperazine in the presence of potassium carbonate under reflux in acetonitrile, giving the target product 2 with a good yield of 75% (Scheme 2).
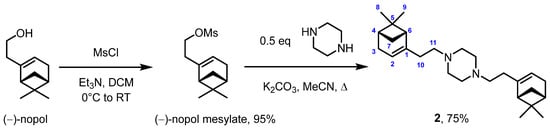
Scheme 2.
Synthesis of 1,4-bis-((−)-nopol)piperazine 2.
The structure of product 2 was determined via 1D and 2D NMR experiments (HSQC, HMBC, and COSY) and HRMS. Moreover, the structure of compound 2 was unambiguously confirmed via an X-ray diffraction analysis of a single crystal (Figure 2).
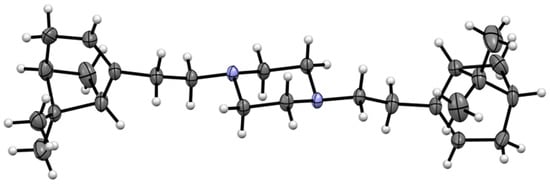
Figure 2.
Structure of compound 2 according to X-ray diffraction data. Thermal ellipsoids of atoms are presented with 30% probability.
There are three crystallographically independent molecules in the crystal unit cell. Two of them are located on rotational 2-fold axes passing through the centers of two C-C piperazine bonds. In the packing of molecules in a crystal (Figure 3), layers of molecules parallel to the bc plane can be distinguished. There are slightly shortened contacts in the layers H...H 2.34 Å and C...H 2.87 Å.
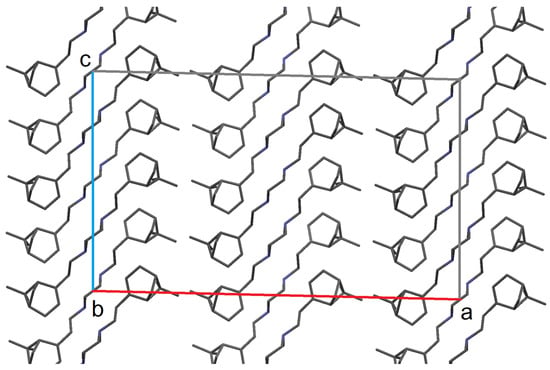
Figure 3.
Crystal packing of compound 2. Hydrogen atoms are not shown.
3. Materials and Methods
3.1. General
(1R)-(−)-Nopol 98% (ee 95%) and other reagents and solvents were purchased from commercial suppliers (Sigma-Aldrich (St. Louis, MO, USA)) and used as received. Column chromatography was performed on silica gel (SiO2; 60–200 µm; Macherey-Nagel (Dueren, Germany)). GC-MS was performed using an Agilent 7890A gas chromatograph (Santa Clara, CA, USA) equipped with a quadrupole mass spectrometer Agilent 5975C as detector; quartz column HP-5MS (copolymer 5% diphenyl and 95% dimethylsiloxane) of length 30 m, internal diameter 0.25 mm, and stationary phase film thickness 0.25 µm. 1H and 13C NMR spectra were recorded using Bruker Avance-III 600 apparatus (Billerica, MA, USA) at 600.30 MHz (1H) and 150.95 MHz (13C). Optical rotation: polAAr 3005 spectrometer (Optical Activity LTD, Huntingdon, UK), CHCl3 soln. HR-MS: DFS-Thermo-Scientific spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) in full scan mode (15–500 m/z, 70 eV electron-impact ionization, direct sample introduction). (−)-Nopol mesylate was synthesized from (−)-nopol according to [3]. X-ray diffraction data for 2 were collected on a Bruker Kappa Apex II diffractometer using Mo Kα radiation. The structure was solved with the ShelXT [10] solution program and the model was refined with ShelXL 2018/3 [11] using full-matrix least-squares minimization on F2.
3.2. Synthesis of 1,4-Bis(2-((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl)ethyl)piperazine 2
A mixture of (−)-nopol mesylate (500 mg, 2.05 mmol), piperazine (88 mg, 1.02 mmol), and K2CO3 (283 mg, 2.05 mmol) in MeCN (20 mL) was refluxed for 4 h. Then, the reaction mixture was washed with brine (3 × 20 mL). The organic layer was dried over anhydrous Na2SO4, and the solvent was evaporated. The residue was subjected to silica gel column chromatography eluting with hexane/EtOAc 100:0→50:50 as mobile phase to obtain compound 2 (588 mg, 75%) as a white solid.
= −34.8 (c 0.48, CHCl3). Tm = 70.2−72.7 °C (EtOAc).
1H NMR (CDCl3, 600 MHz): δ 5.20 (br.s, 2H, H-2), 2.64–2.28 (m, 8H, piperazine), 2.37–2.29 (m, 6H, H-7′, H-11), 2.25–2.10 (m, 8H, H-3, H-10), 2.02–2.06 (m, 2H, H-4), 2.00 (t, J = 5.6 Hz, 2H, H-6), 1.24 (s, 6H, CH3-8), 1.11 (d, J = 8.5 Hz, 2H, H-7), 0.79 (s, 6H CH3-9). 13C NMR (CDCl3, 150 MHz): δ 146.3 (C-1), 116.9 (C-2), 56.7 (C-11), 53.1 (piperazine), 45.9 (C-6), 40.7 (C-4), 37.9 (C-5), 34.3 (C-10), 31.6 (C-7), 31.2 (C-3), 26.2 (C-8), 21.1 (C-9). HRMS: 382.3339 [M]+∙, calcd. 382.3343 (C26H42N2)+∙.
Crystal data of compound 2: C26H42N2, M = 382.61 g·mol−1, crystal size 0.09 × 0.61 × 0.65 mm3, monoclinic, space group C2: a = 29.359(2) Å, b = 9.2406(6) Å, c = 17.5828(15) Å, β = 91.338(3)°, V = 4768.9(6) Å3, Z = 8, Dcalc = 1.066 g/cm3, T = 296 K, 38908 reflections measured (2.32° ≤ 2θ ≤ 54.16°), 10461 unique (Rint = 0.0664). These data were used in all calculations. The final R1 = 0.0601 (I > 2σ(I)) and wR2 = 0.1942 (all data), GOF = 1.018. Largest diff. peak/hole/e Å−3 0.25/−0.27. Data were deposited at the Cambridge Crystallographic Data Centre as CCDC 2324770 (Supplementary Materials). The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/structures.
1H NMR, 13C NMR, and mass spectra of compound 2 are presented in Supplementary Materials.
4. Conclusions
Starting from monoterpene (−)-nopol, a new 1,4-bis(2-((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl)ethyl)piperazine 2 was synthesized. Its structure was determined using 1D and 2D NMR experiments (HSQC, HMBC, and COSY), HRMS, and XRD.
Supplementary Materials
Figure S1: 1H NMR spectra of compound 2 (CDCl3, 600 MHz); Figures S2 and S3: 13C NMR spectrum (BB and JMOD) of compound 2 (CDCl3, 150 MHz); Figure S4: HSQC spectrum of compound 2 (CDCl3, 1H(600 MHz), 13C(150 MHz)); Figure S5: HMBC spectrum of compound 2 (CDCl3, 1H(600 MHz), 13C(150 MHz)); Figure S6: COSY spectrum of compound 2 (CDCl3, 600 MHz); Figure S7: HRMS of compound 2.
Author Contributions
Conceptualization: N.S.L.-Z. and K.P.V.; investigation: N.S.L.-Z., A.D.R. and Y.V.G.; writing—original draft preparation: N.S.L.-Z.; writing—review and editing: K.P.V. and N.F.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Russian Science Foundation (Moscow, Russia) with grant number 22-73-00046.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to acknowledge the Multi-Access Chemical Research Center SB RAS for spectral and analytical measurements.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Salakhutdinov, N.F.; Volcho, K.P.; Yarovaya, O.I. Monoterpenes as a renewable source of biologically active compounds. Pure Appl. Chem. 2017, 89, 1105–1117. [Google Scholar] [CrossRef]
- Li-Zhulanov, N.S.; Zaikova, N.P.; Sari, S.; Gülmez, D.; Sabuncuoğlu, S.; Ozadali-Sari, K.; Arikan-Akdagli, S.; Nefedov, A.A.; Rybalova, T.V.; Volcho, K.P.; et al. Rational Design of New Monoterpene-Containing Azoles and Their Antifungal Activity. Antibiotics 2023, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.K.; Syed, R.; Shin, H.S.; Patel, R.V. Piperazine derivatives for therapeutic use: A patent review (2010–present). Expert Opin. Ther. Pat. 2016, 26, 777–797. [Google Scholar] [CrossRef] [PubMed]
- Shaquiquzzaman, M.; Verma, G.; Marella, A.; Akhter, M.; Akhtar, W.; Khan, M.F.; Tasneem, S.; Alam, M.M. Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents. Eur. J. Med. Chem. 2015, 102, 487–529. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghorbani, M.; Bushra Begum, A.; Zabiulla, Z.; Mamatha, S.V.; Khanum, S.A. Piperazine and morpholine: Synthetic preview and pharmaceutical applications. Res. J. Pharm. Technol. 2015, 8, 611–628. [Google Scholar] [CrossRef]
- Singh, V.; Pacitto, A.; Donini, S.; Ferraris, D.M.; Boros, S.; Illyés, E.; Szokol, B.; Rizzi, M.; Blundell, T.L.; Ascher, D.B.; et al. Synthesis and Structure—Activity relationship of 1-(5-isoquinolinesulfonyl)piperazine analogues as inhibitors of Mycobacterium tuberculosis IMPDH. Eur. J. Med. Chem. 2019, 174, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Protopopova, M.; Hanrahan, C.; Nikonenko, B.; Samala, R.; Chen, P.; Gearhart, J.; Einck, L.; Nacy, C.A. Identification of a new antitubercular drug candidate, SQ109, from a combinatorial library of 1,2-ethylenediamines. J. Antimicrob. Chemother. 2005, 56, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Bogatcheva, E.; Hanrahan, C.; Nikonenko, B.; Samala, R.; Chen, P.; Gearhart, J.; Barbosa, F.; Einck, L.; Nacy, C.A.; Protopopova, M. Identification of new diamine scaffolds with activity against Mycobacterium tuberculosis. J. Med. Chem. 2006, 49, 3045–3048. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).