2-Benzoyl-4-phenyl-1,2,5-thiadiazol-3(2H)-one 1,1-Dioxide
Abstract
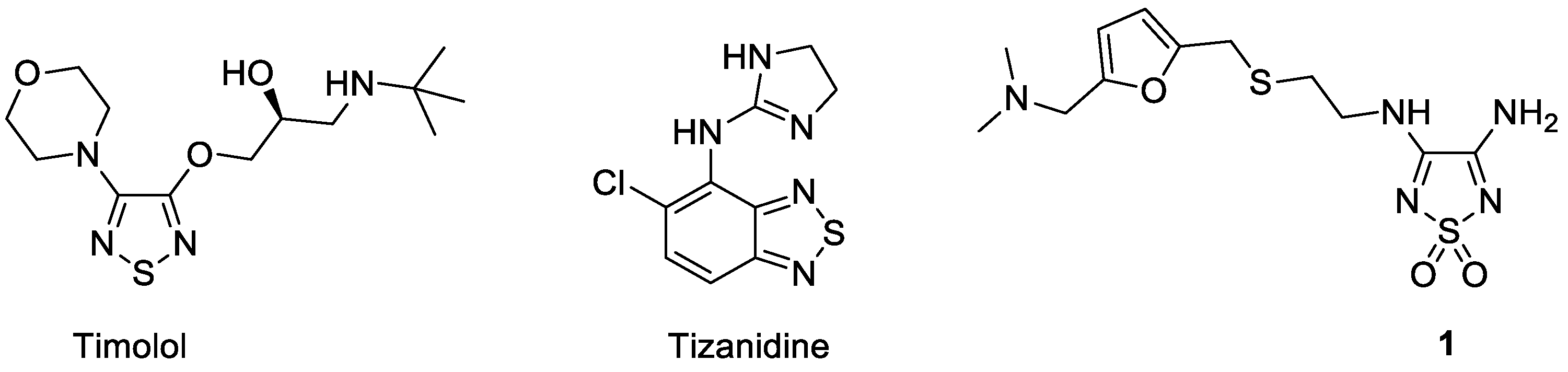
1. Introduction
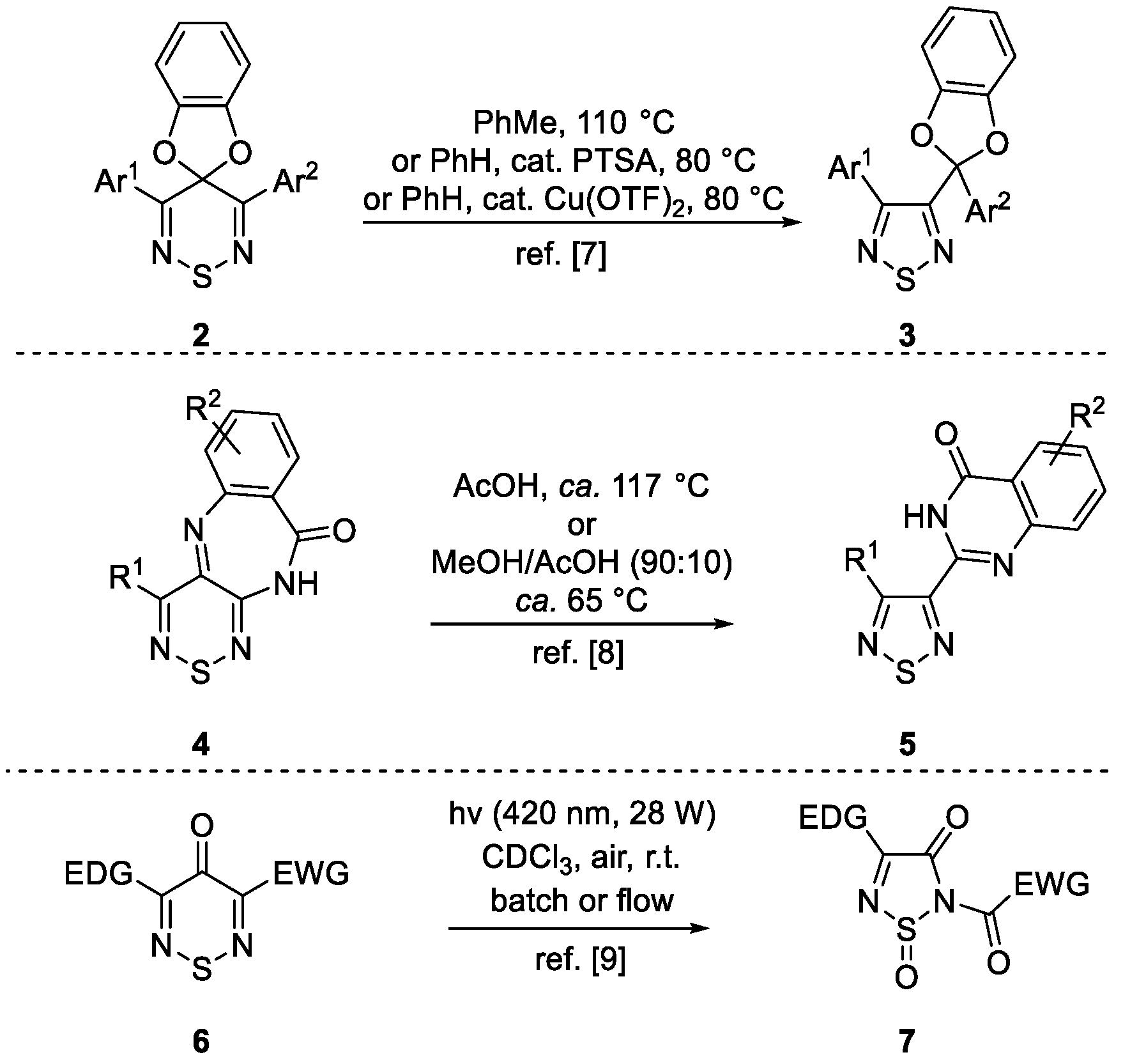
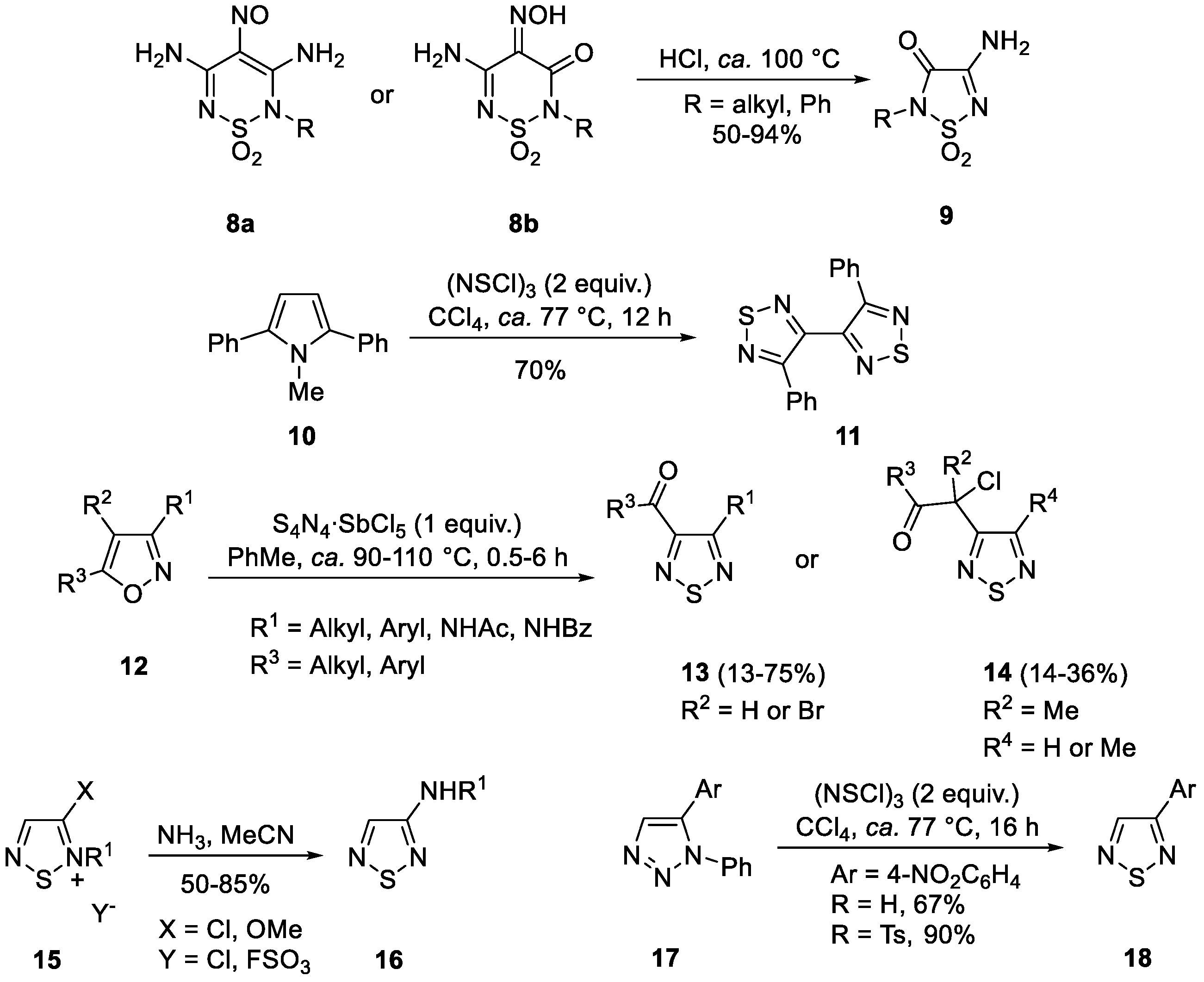
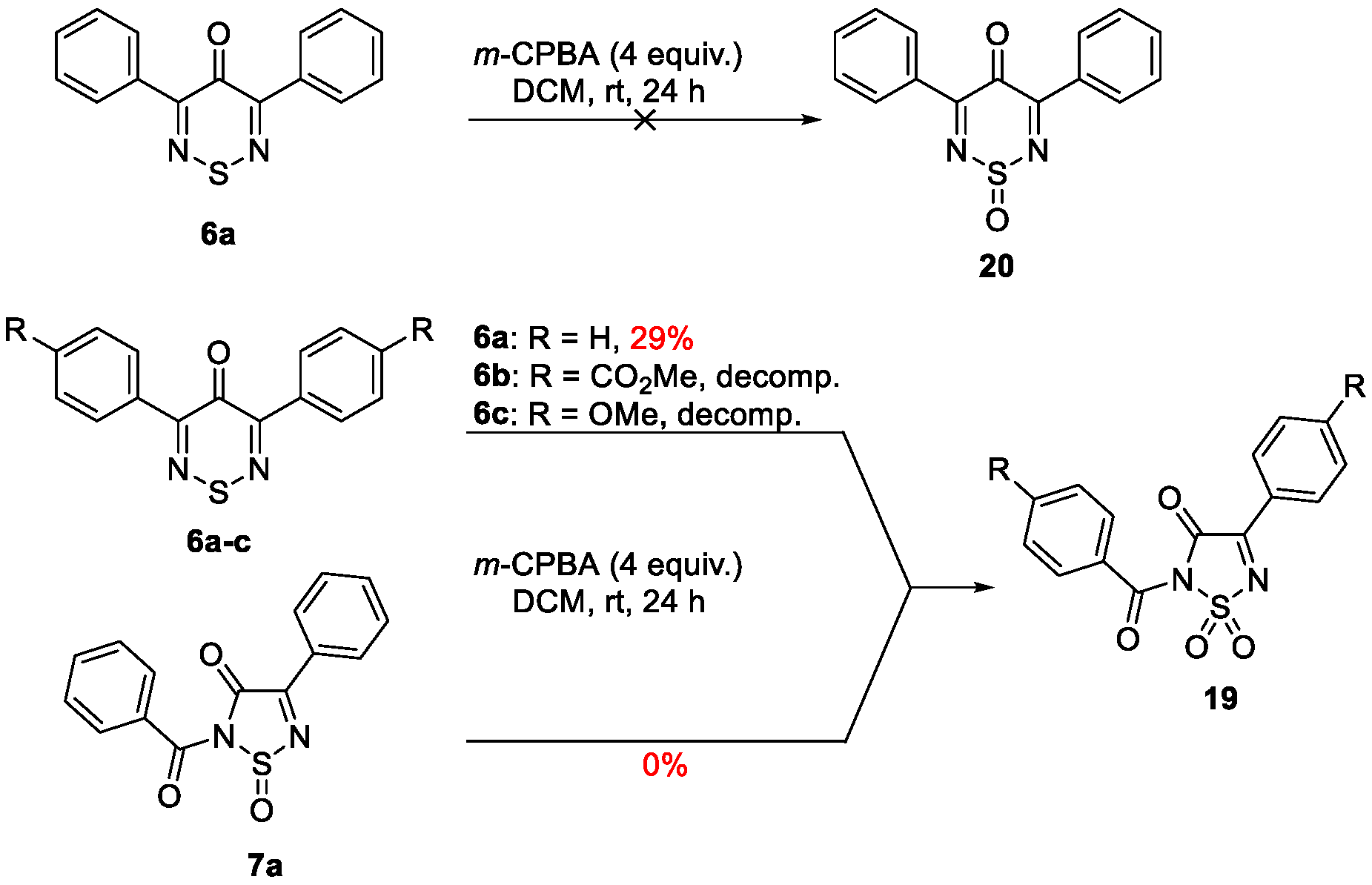
2. Results and Discussion
3. Materials and Methods
2-Benzoyl-4-phenyl-1,2,5-thiadiazol-3(2H)-one 1,1-dioxide (19)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rakitin, O.A. 1,2,5-Thiadiazoles. In Comprehensive Heterocyclic Chemistry IV; Koutentis, P.A., Black, D., Cossy, J., Stevens, C.V., Eds.; Elsevier: Oxford, UK, 2022; Volume 5, Chapter 5.09; pp. 371–406. [Google Scholar] [CrossRef]
- Koutentis, P.A. 1,2,5-Thiadiazoles. In Comprehensive Heterocyclic Chemistry III; Zhdankin, V.V., Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 5, Chapter 5.09; pp. 515–565. [Google Scholar] [CrossRef]
- Wang, X.; Lawson, J.D.; Marx, M.A.; Smith, C.R.; Kuluk, S. Azaquinazoline Pan-Kras Inhibitors. WO 2022/132200, 23 June 2022. [Google Scholar]
- Han, Y.; Achab, A.; Biju, P.; Deng, Y.; Fradera, X.; Guo, L.; He, S.; Kozlowski, J.; Kurulasuriya, R.; Liu, K.; et al. Compounds as Indoleamine 2,3-Dioxygenase Inhibitors. US 10538497, 21 January 2020. [Google Scholar]
- Algieri, A.A.; Luke, G.M.; Standridge, R.T.; Brown, M.; Partyka, R.A.; Crenshaw, R.R. 1,2,5-Thiadizole 1-oxide and 1,1-dioxide derivatives. A new class of potent histamine H2-receptor antagonists. J. Med. Chem. 1982, 25, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Koutentis, P.A. The Chemistry of Non-S-oxidised 4H-1,2,6-Thiadiazines. Targets Heterocycl. Syst. 2018, 22, 82–118. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Kourtellaris, A.; Koutentis, P.A. The Acid and/or Thermal Mediated Ring Contraction of 4H-1,2,6-Thiadiazines to Afford 1,2,5-Thiadiazoles. Org. Lett. 2016, 18, 4056–4059. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Kourtellaris, A.; Koutentis, P.A. Synthesis and Chemistry of Benzo[e][1,2,6]thiadiazino[3,4-b][1,4]diazepin-10(11H)-ones and Related Ring Transformations. J. Org. Chem. 2021, 86, 5702–5713. [Google Scholar] [CrossRef] [PubMed]
- Broumidis, E.; Thomson, C.G.; Gallagher, B.; Sotorríos, L.; McKendrick, K.G.; Macgregor, S.A.; Paterson, M.J.; Lovett, J.E.; Lloyd, G.O.; Rosair, G.M.; et al. The Photochemical Mediated Ring Contraction of 4H-1,2,6-Thiadiazines to Afford 1,2,5-Thiadiazol-3(2H)-one 1-Oxides. Org. Lett. 2023, 25, 6907–6912. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Garcıa-Gomez, C.; de Paz, J.L.G.; Jimeno, M.L.; Aran, V.J. Synthesis, hydrolysis reactions and conformational study of 2-substituted 3,5-diamino-4-nitroso-2H-1,2,6-thiadiazine 1,1-dioxides. J. Chem. Soc. Perkin Trans. 2 1996, 293–297. [Google Scholar] [CrossRef]
- Duan, X.-G.; Rees, C.W. Conversion of pyrroles into bi-1,2,5-thiadiazoles: A new route to biheterocycles. J. Chem. Soc. Perkin Trans. 1 1997, 3189–3196. [Google Scholar] [CrossRef]
- Kim, K.-J.; Kim, K. Reactions of 5-substituted 3-alkyl- and 3-aryl-isoxazoles with tetrasulfur tetranitride antimony pentachloride complex (S4N4·SbCl5): Complete regioselective formation of 4-substituted 3-acyl- and 3-aroyl-1,2,5-thiadiazoles and their mechanism of formation. J. Chem. Soc. Perkin Trans. 1 1998, 2175–2180. [Google Scholar] [CrossRef]
- Rokach, J.; Hamel, P.; Girard, Y.; Reader, G. General method for the preparation of N-monosubstituted 3-isothiazole and 3-(1,2,5-thiadiazole)amines. Preparation of a new class of 2-substituted 1,2,5-thiadiazol-3(2H)-ones. J. Org. Chem. 1979, 44, 1118–1124. [Google Scholar] [CrossRef]
- Rees, C.W.; Yue, T.-Y. Conversion of enamines, enamides and triazoles by trithiazyl trichloride into 1,2,5-thiadiazoles. J. Chem. Soc. Perkin Trans. 1 2001, 662–667. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Chemistry of 4-Dicyanomethylene-1,2,6-thiadiazines. J. Chem. Soc. Perkin Trans. 1 2000, 1081–1088. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Kourtellaris, A.; Koutentis, P.A. Oxidations of 4H-1,2,6-Thiadiazines. ChemistrySelect 2022, 7, e202204204. [Google Scholar] [CrossRef]
- Bruker. Apex3, Saint; Bruker AXS Inc.: Madison, WI, USA, 2018. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Kizas, C.; Koutentis, P.A. Palladium Catalyzed C–C Coupling Reactions of 3,5-Dichloro-4H-1,2,6-thiadiazin-4-one. Org. Lett. 2011, 13, 3466–3469. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broumidis, E.; Patterson, S.B.H.; Rosair, G.M.; Koutentis, P.A.; Kalogirou, A.S. 2-Benzoyl-4-phenyl-1,2,5-thiadiazol-3(2H)-one 1,1-Dioxide. Molbank 2024, 2024, M1774. https://doi.org/10.3390/M1774
Broumidis E, Patterson SBH, Rosair GM, Koutentis PA, Kalogirou AS. 2-Benzoyl-4-phenyl-1,2,5-thiadiazol-3(2H)-one 1,1-Dioxide. Molbank. 2024; 2024(1):M1774. https://doi.org/10.3390/M1774
Chicago/Turabian StyleBroumidis, Emmanouil, Samuel B. H. Patterson, Georgina M. Rosair, Panayiotis A. Koutentis, and Andreas S. Kalogirou. 2024. "2-Benzoyl-4-phenyl-1,2,5-thiadiazol-3(2H)-one 1,1-Dioxide" Molbank 2024, no. 1: M1774. https://doi.org/10.3390/M1774
APA StyleBroumidis, E., Patterson, S. B. H., Rosair, G. M., Koutentis, P. A., & Kalogirou, A. S. (2024). 2-Benzoyl-4-phenyl-1,2,5-thiadiazol-3(2H)-one 1,1-Dioxide. Molbank, 2024(1), M1774. https://doi.org/10.3390/M1774







