Abstract
In this study, we outline the eco-friendly mechanosynthesis of N-(2,2-diphenylethyl)-4-nitrobenzamide by reacting 2,2-diphenylethan-1-amine with 4-nitrobenzoyl chloride. The resulting bio-functional hybrid compound was meticulously characterized through the analysis of 1H-, 13C-NMR, UV, and detailed mass spectral analysis.
1. Introduction
2,2-Diphenylethan-1-amine (Figure 1), commonly referred to as diphenylethylamine, finds diverse applications in various fields. Its significance lies in its utility as a key building block in organic synthesis. As an amine derivative, it serves as a versatile intermediate for the synthesis of pharmaceuticals, agrochemicals, and specialty chemicals. The unique structural features of 2,2-diphenylethan-1-amine make it valuable for the creation of bioactive compounds, contributing to the development of new drugs and chemical entities. Additionally, its involvement in the synthesis of hybrid materials and polymers further extends its applications, showcasing its importance in both medicinal chemistry and material science [1,2,3,4,5,6].
Figure 1.
Structural formulas of 2,2-diphenylethan-1-amines.
For example, 2,2-diphenylethylamine serves as a cornerstone in the synthesis of 4-aryl-1,2,3,4-tetrahydroisoquinoline derivatives, facilitating the creation of diverse molecular frameworks with potential therapeutic applications, inspired by the natural the alkaloids Cherylline, Latifine, Nomefensine, Dichlofensine, and others [7].
The nitro group holds a significant role in pharmaceutical chemistry, contributing to the design and development of various drugs with therapeutic benefits. A significant number of compounds employed in medical practice feature a nitro group [8]. Its importance stems from its unique chemical properties and diverse biological activities. The nitro group can serve as a pharmacophore, imparting specific physiological effects to a molecule.
In medicinal chemistry, the nitro group is often strategically incorporated into drug structures to enhance bioactivity. Nitro-containing compounds have shown anti-microbial, anti-parasitic, anti-inflammatory, and anti-cancer properties. The ability of the nitro group to undergo metabolic reduction within the body, leading to the formation of reactive intermediates, is a key factor in the pharmacological activity of these compounds [9,10,11].
Moreover, the nitro group can modulate the pharmacokinetics of a drug, influencing factors such as absorption, distribution, metabolism, and excretion. The presence of the nitro group can also facilitate prodrug strategies, allowing for controlled release of the active form within the body. The electron-withdrawing effect in aromatic rings, induced by resonance with the nitro group, deactivates specific positions and alters molecular polarity. This facilitates interactions with nucleophilic sites in proteins, causing inhibitory effects. The nitro group acts as both a pharmacophore and a toxicophore, adding complexity for medicinal chemists. While its presence may not directly correlate with activity, it significantly influences pharmacokinetics [12].
The mutagenic risk associated with nitroarenes makes them less favored in drug design. The presence and positioning of the nitro group on aromatic rings influences the mutagenic profile of compounds. Nitro reduction plays a crucial role, forming major DNA adducts with varying rates of formation and preferential sites. Each mutagen carries a unique signature of mutagenic specificity, leading to diverse effects. Notably, not every nitro-containing drug is mutagenic, as seen in anti-tubercular drug discovery [13].
Connecting 2,2-diphenylethan-1-amine with 4-nitrobenzoyl chloride is crucial for their joint application in organic synthesis and pharmaceutical development. The former, a versatile building block, contributes to bioactive compound synthesis for drug development, while the latter, with its nitro group, plays a key role in enhancing bioactivity in pharmaceutical chemistry. The strategic incorporation of the nitro group into drug structures results in compounds with diverse therapeutic benefits. This connection facilitates the synthesis of hybrid materials and polymers, demonstrating their collective importance in medicinal chemistry. The electron-withdrawing effect induced by the nitro group influences molecular interactions and pharmacokinetics, underlining the significance of linking these compounds for the development of novel drugs and chemical entities. Understanding mutagenic risks associated with nitroarenes is vital in drug design, underscoring the importance of connecting these compounds for a comprehensive approach in pharmaceutical development.
2. Results and Discussion
Herein, we report the successful synthesis of N-(2,2-diphenylethyl)-4-nitrobenzamide 3, as shown in Scheme 1. The synthesis of the novel hybrid molecule was achieved through the mechanochemical method using a shaker type ball mill. In recent years, there has been a growing use of mechanochemistry as a solvent-free synthetic approach for producing a wide range of organic compounds. This method offers an environmentally friendly, safer, and more efficient transformation pathway, resulting in larger-scale yields of the desired products [14,15].
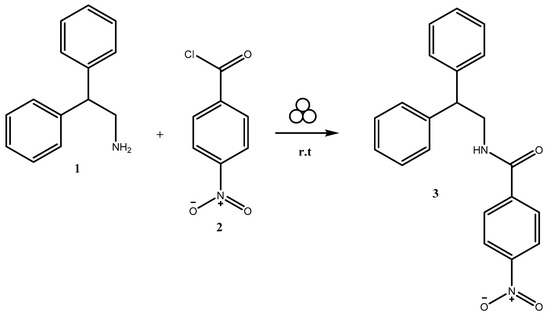
Scheme 1.
Mechanochemical synthesis of compound 3.
For this purpose, 2,2-diphenylethan-1-amine 1 (0.1 mmol) and 4-nitrobenzoyl chloride (0.1 mmol) were placed in a 4 mL stainless steel milling jar, along with a 10 mm stainless steel ball. After ball-milling the reaction mixture for 5 min at room temperature, the inspected TLC demonstrated the acquisition of the final product 3 (Scheme 1).
Synthesized molecule 3 is a new molecule (Reaxis) and is fully characterized with its melting point and with 1H-, 13C-NMR, UV, and detailed mass spectral analysis. The new amide molecule 3 is fragmented with cleavage of the amide bond, generating characteristic diphenylmethylium and nitrophenylacyl fragment ions. Obtaining them is related to their fragmentation by pathway 1, 2, and 3 (Figure 2).
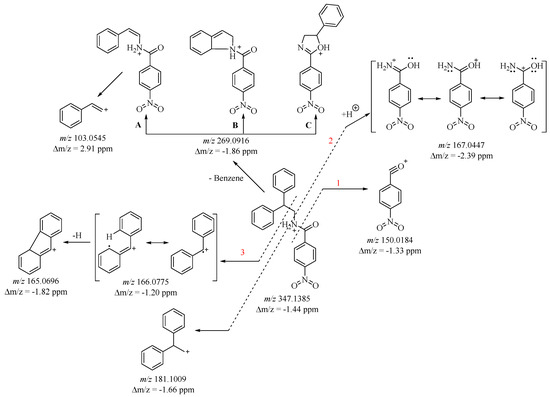
Figure 2.
Proposed fragmentation of protonated compound 3.
Path 1 is associated with cleavage of the NH-C(O) bond, and leads to the formation of a stable (4-nitrobenzylidyne)oxonium cation (C7H4NO3+) with m/z 150 (Figure 2). Ions m/z 167 and 181 result from N-C bond cleavage (pathway 2), and correspond to a protonated amide (C7H7N2O3+) cation and a 2,2-diphenylethan-1-ylium (C14H13+) cation, respectively (Figure 2 and Figure S5). The m/z 181 ion exhibits the highest intensity, attributed to its distinctly aromatic character. Path 3 involves the beta fragmentation of amides, leading to the formation of the resonance-stable diphenylmethylium cation-radical with a mass-to-charge ratio (m/z) of 166. Additionally, upon closer examination, m/z 166 could also correspond to a p-nitrobenzylamide cation radical. When data are presented using nominal masses, both possibilities can be entertained. However, employing High-Resolution Mass Spectrometry (HRMS) enables precise analysis of fragment masses, facilitating the identification of the specific fragment generated. Analysis confirms the presence of the characteristic diphenylmethylium cation radical with a mass error of less than 2 ppm. The fragment ion at m/z 165 arises from the cleavage of a hydrogen cation followed by the cyclization of a diphenylmethylium fragment (refer to Figure 2). Apart from the amide bond cleavage, the molecular ion (m/z 347) experiences the elimination of a benzyl group under ESI-MS conditions. This process leads to the formation of a fragment with m/z 269. There are multiple ways through which it can be attained. Following an exhaustive examination of the mass spectrum, three potential pathways are identified—Path A, B, and C (refer to Figure 3).
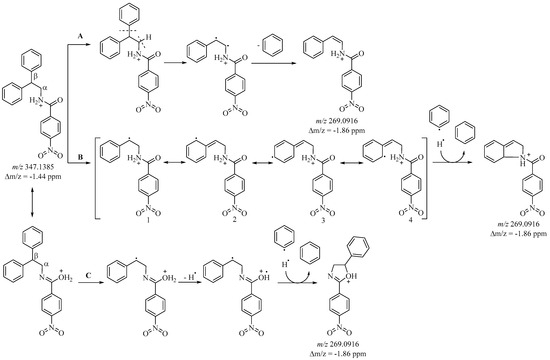
Figure 3.
Possible pathways for obtaining fragment ion m/z 269.
Path A involves the cleavage of sigma bonds at the alpha and beta C-atoms, resulting in the detachment of a benzyl and hydrogen radical. The combination of these radicals yields a benzene molecule. Furthermore, a biradical cation is formed, which subsequently undergoes rearrangement into a resonance-stabilized cation (see Figure 3). Subsequent cleavage of the N-C bond in this fragment results in the generation of a stable aromatic ion with m/z 103 (refer to Figure 2 and Figure S5).
Under MS/MS conditions, Routes B and C result in the generation of resonance-stabilized cyclic cations. Both pathways involve the detachment of the benzyl radical from the β C-atom. In Route B, subsequent to the separation of the benzylic fragment, a radical cation emerges, exhibiting stabilization through four possible canonical structures (see Figure 3). It is probable that canonical structure 4 is more favorable for initiating a new cycle by cleaving the hydrogen radical from the amide group, yielding a stable ion with an m/z of 269 (see Figure 3). Formation of the same ion via Route C occurs through closure between the β C-atom and the O-atom of the amide group.
3. Materials and Methods
All reagents and chemicals were acquired from commercial sources (Sigma-Aldrich S.A. and Riedel-de Haën, Sofia, Bulgaria) and used without further purification. NMR spectral data were recorded on a Bruker Avance Neo 400 spectrometer (BAS-IOCCP—Sofia, Bruker, Billerica, MA, USA) at 400 MHz for 1H NMR and 101 MHz for 13C NMR. The spectra were obtained in DMSO-d6, with chemical shifts reported in relative ppm referenced to tetramethylsilane (TMS) (δ = 0.00 ppm) as an internal standard, and coupling constants indicated in Hz. The NMR recordings were performed at room temperature (approximately 295 K). Melting points were determined using a Boetius hot stage apparatus and are uncorrected. Absorbance measurements were conducted with a Camspec M508 spectrophotometer, Leeds, UK. MS analysis was carried out on a Q Exactive Plus high-resolution mass spectrometer (HRMS) with a heated electrospray ionization source (HESI-II) from Thermo Fisher Scientific, Inc., Bremen, Germany, equipped with a Dionex Ultimate 3000RSLC ultrahigh-performance liquid chromatography (UHPLC) system (Thermo Fisher Scientific, Inc., Waltham, MA, USA). TLC was performed on 0.2 mm Fluka silica gel 60 plates (Merck KGaA, Darmstadt, Germany). Mechanosynthesis was conducted using a ball mill Vibrator model NARVA Erbisdorf, DDR-GM9458, with power specifications of 220/110 V, 30 W, 50 Hz, and a degree of protection rated as P21. The working chamber had a volume of 4 mL, and the synthesis utilized two stainless steel balls, each measuring 10 mm in diameter and weighing 4 g.
3.1. Synthesis of N-(2,2-diphenylethyl)-4-nitrobenzamide
2,2-Diphenylethan-1-amine 1 (0.1 mmol, 0.0197 g) and 4-nitrobenzoyl chloride (0.1 mmol, 0.0185 g) were placed in a 4 mL stainless steel milling jar, along with two 10 mm stainless steel balls. The ball mill was set to vibrate at a frequency of 50 Hz for 5 min. Subsequent to that, the milling jar contents underwent a double wash with dichloromethane (10 mL) and water (5 mL) each, leading to the separation of the two layers. The aqueous layer was then subjected to extraction with dichloromethane (2 × 10 mL), and the combined organic fractions were dried using anhydrous Na2SO4 before being concentrated under vacuum.
3.2. N-(2,2-diphenylethyl)-4-nitrobenzamide
White solid (m.p. 190–192 °C), yield 89% (0.0307 g) 1H NMR (400 MHz, DMSO-d6) δ 8.89 (t, J = 5.5 Hz, 1H), 8.32–8.24 (m, 2H), 7.97–7.89 (m, 2H), 7.32–7.27 (m, 7H), 7.24–7.13 (m, 3H), 4.44 (t, J = 7.9 Hz, 1H), 3.95 (dd, J = 8.1, 5.5 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 165.17 (C=O), 149.40 (C-Ar), 143.19 (C-Ar), 140.60 (C-Ar), 129.03 (C-Ar), 128.91 (C-Ar), 128.39 (C-Ar), 126.87 (C-Ar), 123.97 (C-Ar), 50.23 (CH2), 44.44 (CH). UV λmax, MeOH: 239 (ε = 4970) nm, 289 (ε = 2800) nm. HRMS Electrospray ionization (ESI) m/z calcd for [M + H]+ C21H19N2O3+ = 347.1390, found 347.1385 (mass error ∆m = −1.44 ppm).
Supplementary Materials
The following supporting information can be downloaded online: Figure S1: 1H-NMR spectrum of compound 3; Figure S2: 13C-NMR spectrum of compound 3; Figure S3: UV spectrum of compound 3; Figure S4: ESI-HRMS of compound 3; Figure S5: Mass spectrum of compound 3 obtained by positive ion ESI-MS/MS.
Author Contributions
Conceptualization, I.I. and S.M.; methodology, S.M. and D.B.; software, S.M. and D.B.; validation, I.I., S.M. and D.B.; formal analysis, S.M., D.B., P.N., D.D. and L.K.; investigation, S.M. and D.B.; resources, I.I.; data curation, I.I. and S.M.; writing—original draft preparation, S.M.; writing—review and editing, S.M., D.B. and I.I.; visualization, S.M. and D.B.; supervision, I.I.; project administration, S.M.; funding acquisition, I.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Plovdiv University “Paisii Hilendarski”, grant number: ΦΠ23-XΦ-005.
Data Availability Statement
The data presented in this study are available in this article and supporting Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Przybyla, M.; Yilmaz, G.; Becer, R. Natural cyclodextrins and their derivatives for polymer synthesis. Polym. Chem. 2020, 11, 7582–7602. [Google Scholar] [CrossRef]
- Martinez-Cuezva, A.; Saura-Sanmartin, A.; Nicolas-Garcia, T.; Navarro, C.; Orenes, R.-A. Photoswitchable interlocked thiodiglycolamide as a cocatalyst of a chalcogeno-Baylis-Hillman reaction. Chem. Sci. 2017, 8, 3775–3780. [Google Scholar] [CrossRef]
- Pervez, H.; Ahmad, M.; Zaib, S.; Yaqub, M.; Naseer, M.; Iqbal, J. Synthesis, cytotoxic and urease inhibitory activities of some novel isatin-derived bis-Schiff bases and their copper (II) complexes. Med. Chem. Commun. 2016, 7, 914–923. [Google Scholar] [CrossRef]
- Xiang, J.-J.; Huang, C.; Wang, H.-J.; Li, F.-B.; Shi, J.-L.; Huang, Y.-S.; Liu, L.; Liu, C.-Y.; Asiri, A.; Alamry, K. Metal-free-mediated synthesis of fulleropyrrolines by the reaction of [60]fullerene with β-substituted ethylamines. New J. Chem. 2017, 41, 8725–8728. [Google Scholar] [CrossRef]
- Pippione, A.; Sainas, S.; Goyal, P.; Fritzson, I.; Cassiano, G.; Giraudo, A.; Giorgis, M.; Tavella, T.; Bagnati, R.; Rolando, B.; et al. Hydroxyazole scaffold-based Plasmodium falciparum dihydroorotate dehydrogenase inhibitors: Synthesis, biological evaluation and X-ray structural studies. Eur. J. Med. Chem. 2019, 163, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Sainas, S.; Pippione, A.; Boschi, D.; Lolli, M. Hydroxyazoles as acid isosteres and their drug design applications-Part 1: Monocyclic systems. In Advances in Heterocyclic Chemistry, 1st ed.; Meanwell, N., Lolli, M., Eds.; Academic Press; Elsevier Inc.: Cambridge, MA, USA, 2021; Volume 134, pp. 185–272. [Google Scholar] [CrossRef]
- Manolov, S.; Atanasova, S.; Ghate, M.; Ivanov, I. A brief review of Cherylline synthesis. Indian J. Chem. Sect. B 2015, 54B, 1301–1320. Available online: https://nopr.niscpr.res.in/bitstream/123456789/33031/3/IJCB%2054B%289%29%201301-1320.pdf (accessed on 2 January 2024).
- Nepali, K.; Lee, H.-Y.; Liou, J.-P. Nitro-Group-Containing Drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef] [PubMed]
- Tavera-Hernández, R.; Jiménez-Estrada, M.; Alvarado-Sansininea, J.; Nieto-Camacho, A.; López-Muñoz, H.; Sánchez-Sánchez, L.; Escobar, M. Synthesis of Chrysin, Quercetin and Nariginin Nitroderivatives: Antiproliferative, Anti-inflammatory and Antioxidant Activity. Lett. Drug Des. Discov. 2021, 18, 795–805. [Google Scholar] [CrossRef]
- Ribeiro, T.; Machado-Ferreira, E.; Guimarães, L.; Cavaleiro, J.; Britto, A.; Redua, N.; Pereira de Souza, L.; Pimentel, A.; Picciani, P.; Oliveira, O., Jr.; et al. Novel cytotoxic amphiphilic nitro-compounds derived from a synthetic route for paraconic acids. Colloids Surf. 2021, 626, 126984. [Google Scholar] [CrossRef]
- Toro, P.; Acuña, A.; Mallea, M.; Lapier, M.; Moncada-Basualto, M.; Cisterna, J.; Brito, I.; Klahn, H. Condensation and substitution products obtained in reactions of isomeric bromonitrofuraldehydes with ferrocenylamine: Electrochemistry and anti-parasitic evaluation. J. Organomet. Chem. 2019, 901, 120946. [Google Scholar] [CrossRef]
- Noriega, S.; Cardoso-Ortiz, J.; López-Luna, A.; Cuevas-Flores, M.; De La Torre, J. The Diverse Biological Activity of Recently Synthesized Nitro Compounds. Pharmaceuticals 2022, 15, 717. [Google Scholar] [CrossRef] [PubMed]
- Purohit, V.; Basu, A. Mutagenicity of Nitroaromatic Compounds. Chem. Res. Toxicol. 2000, 13, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Margetić, D. Recent applications of mechanochemistry in synthetic organic chemistry. Pure Appl. Chem. 2023, 93, 315–328. [Google Scholar] [CrossRef]
- Zhu, L.; Deng, L.; Xie, Y.; Liu, L.; Ma, X.; Liu, R. Mechanochemistry, solvent-free and scale-up: Application toward coupling of acids and amines to amides. Results Chem. 2023, 5, 100882. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).