(Z)-1-Benzyl-5-(4-bromophenyl)-5-hydroxy-4-(2-oxomorpholin-3-ylidene)pyrrolidine-2,3-dione
Abstract
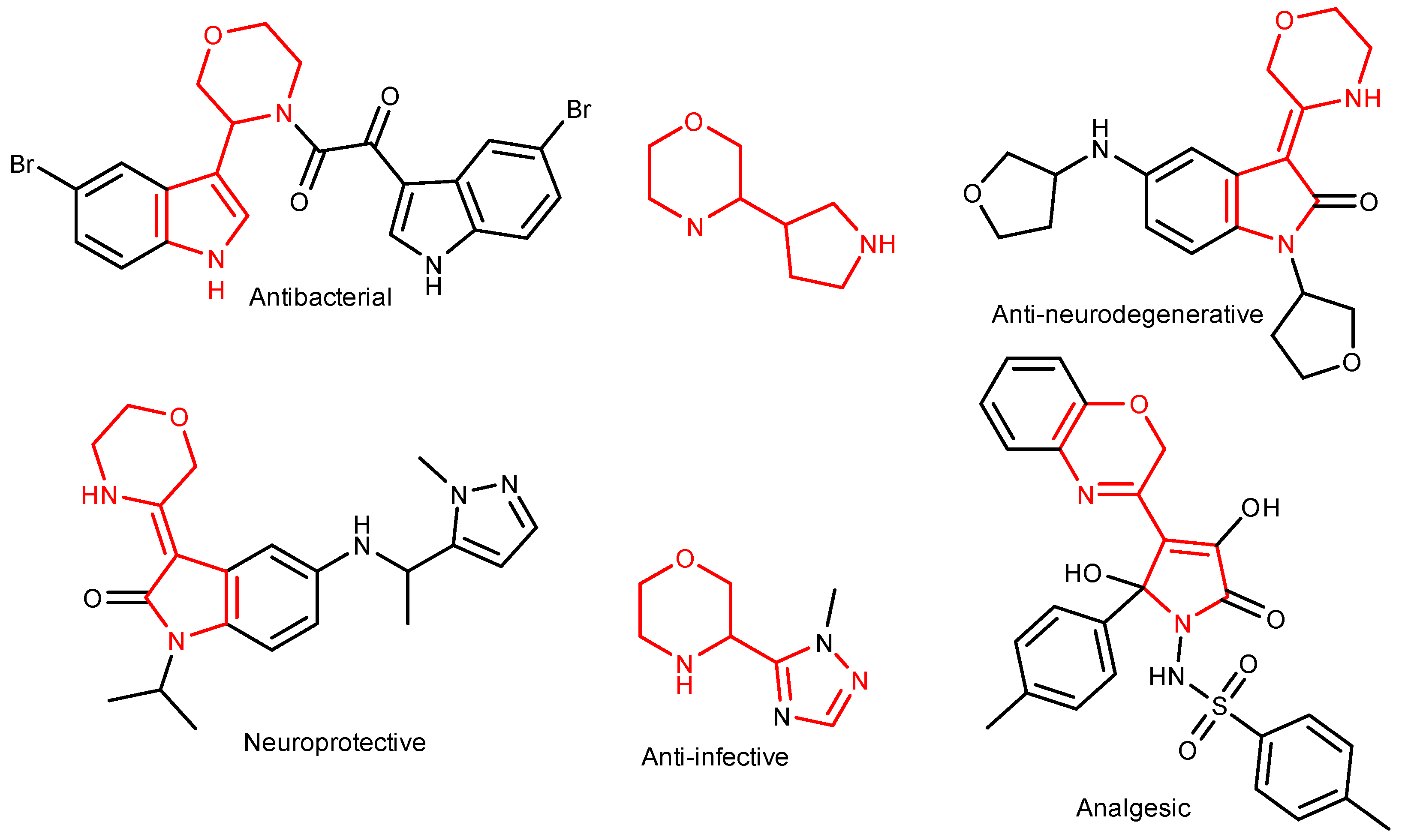
:1. Introduction
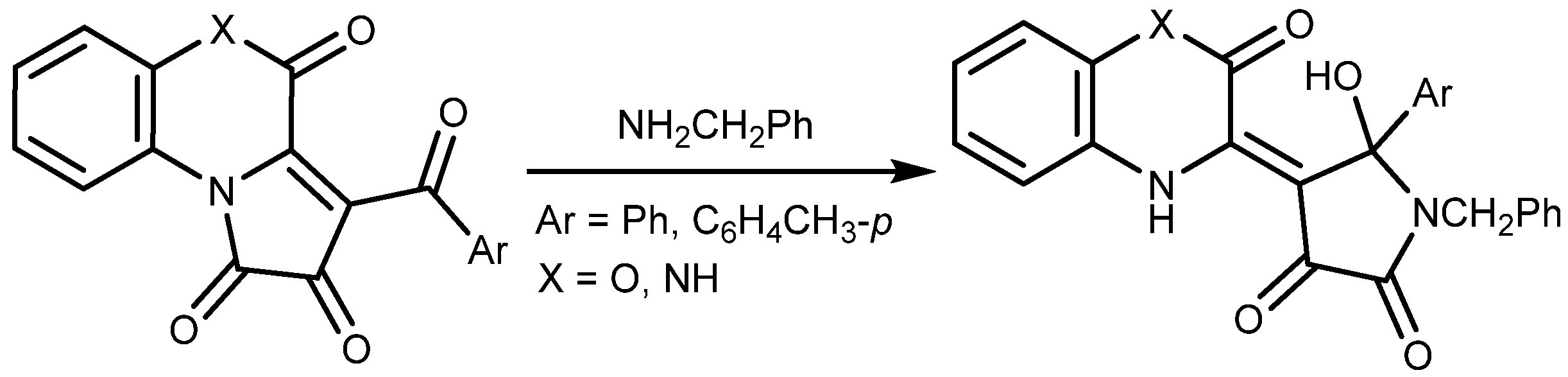
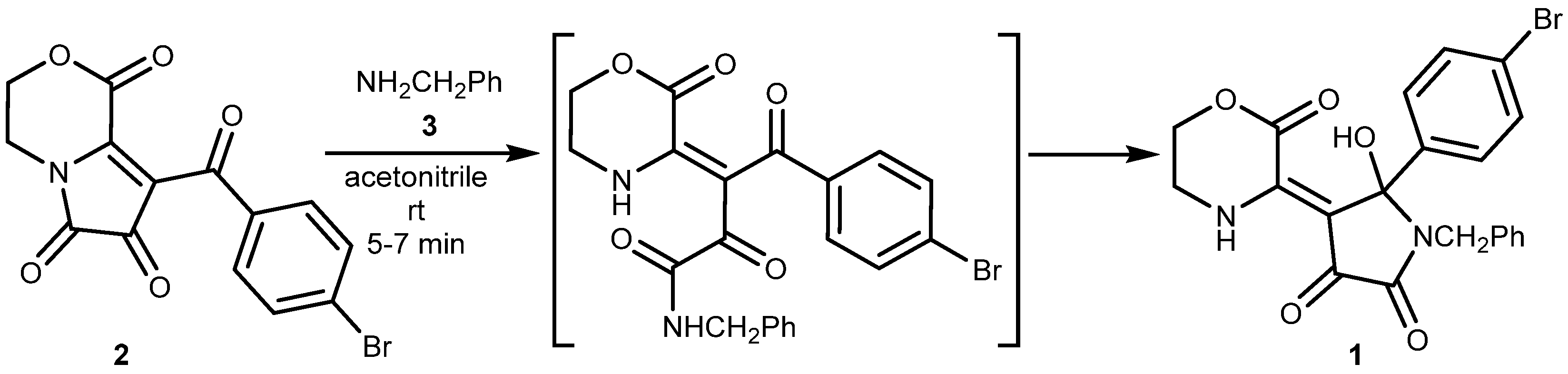
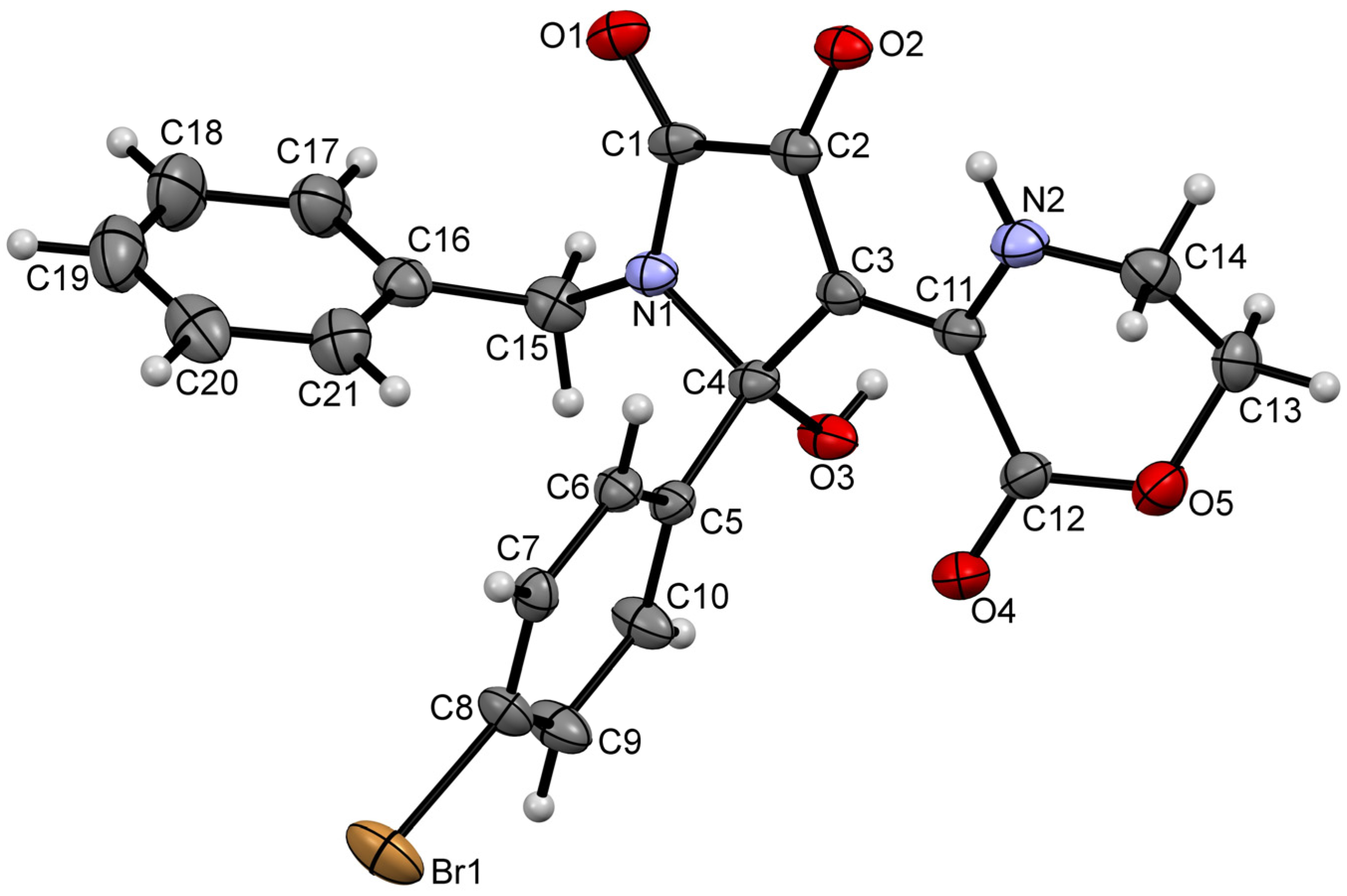
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. (Z)-1-Benzyl-5-(4-bromophenyl)-5-hydroxy-4-(2-oxomorpholin-3-ylidene)pyrrolidine-2,3-dione 1
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denis, J.-N.; Jolivalt, M.C.; Maurin, L.M.; Burchak, N.O. Bis-Indolic Derivatives, Their Uses in Particular as Antibacterials. Patent US 20,140,228,359, 14 August 2014. [Google Scholar]
- Gerlach, K.; Eickmeier, C.; Kriegl, J.M.; Kussmaul, L.; Rudolf, K.; Schmid, B. Modulators of Complex I. Patent WO 2,022,171,265, 18 August 2022. [Google Scholar]
- Gerlach, K.; Eickmeier, C.; Kriegl, J.M.; Kussmaul, L.; Rudolf, K.; Schmid, B. Preparation of Aminoindolone Derivatives for Use as Mitochondrial Complex I Modulators. Patent US 20,210,061,761, 4 March 2021. [Google Scholar]
- Shanina, E.; Kuhaudomlarp, S.; Lal, K.; Seeberger, P.H.; Imberty, A.; Rademacher, C. Druggable Allosteric Sites in β-Propeller Lectins. Angew. Chem. Int. Ed. 2022, 61, e202109339. [Google Scholar] [CrossRef] [PubMed]
- Suchkova, N.V.; Maslivets, A.N.; Makhmudov, R.R.; Mashevskaya, I.V. 2-Aryl-2,4-dihydroxy-2,5-dihydro-3-heteroaryl-5-oxo-1H-pyrrol-1-yl-4-methyl Benzene-sulfanol Amides with Analgesic Activity. Patent RU 2,626,650, 6 March 2017. [Google Scholar]
- Aliev, Z.G.; Maslivets, A.N.; Mashevskaya, I.V.; Andreichikov, Y.S.; Atovmyan, L.O. Interaction of 3-p-toluoyl-1,2-dihydro-4H-pyrrolo[2,1-c][1,4]benzoxazine-1,2,4-trione with benzylamine. Synthesis and crystal and molecular structure of Z-3-(1-benzyl-2-hydroxy-4,5-dioxo-2-p-tolyltetrahydropyrrol-3-idene)-3,4-dihydro-2H-1,4-benzoxazin-2-one. Russ. Chem. Bull. 1997, 46, 546–549. [Google Scholar] [CrossRef]
- Mashevskaya, I.V.; Kol’tsova, S.V.; Maslivets, A.N. An Unusual Recyclization of a Substituted Pyrrolo[1,2-a]quinoxaline-1,2,4-trione under the Action of Benzylamine. Chem. Het. Comp. 2000, 36, 1355–1356. [Google Scholar] [CrossRef]
- CrysAlisPro. Version 1.171.37.33 (Release 27-03-2014 CrysAlis171.NET). Agilent Technologies, Oxford Diffraction: Wroclaw, Poland. 2014. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 28 November 2023).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment–Olex2 dissected. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tretyakov, N.A.; Maslivets, A.N. (Z)-1-Benzyl-5-(4-bromophenyl)-5-hydroxy-4-(2-oxomorpholin-3-ylidene)pyrrolidine-2,3-dione. Molbank 2023, 2023, M1751. https://doi.org/10.3390/M1751
Tretyakov NA, Maslivets AN. (Z)-1-Benzyl-5-(4-bromophenyl)-5-hydroxy-4-(2-oxomorpholin-3-ylidene)pyrrolidine-2,3-dione. Molbank. 2023; 2023(4):M1751. https://doi.org/10.3390/M1751
Chicago/Turabian StyleTretyakov, Nikita A., and Andrey N. Maslivets. 2023. "(Z)-1-Benzyl-5-(4-bromophenyl)-5-hydroxy-4-(2-oxomorpholin-3-ylidene)pyrrolidine-2,3-dione" Molbank 2023, no. 4: M1751. https://doi.org/10.3390/M1751
APA StyleTretyakov, N. A., & Maslivets, A. N. (2023). (Z)-1-Benzyl-5-(4-bromophenyl)-5-hydroxy-4-(2-oxomorpholin-3-ylidene)pyrrolidine-2,3-dione. Molbank, 2023(4), M1751. https://doi.org/10.3390/M1751






