Abstract
The present paper describes the preparation and characterization of a new dinuclear ligand based on terpyridine featuring a diselenide unit. This new compound was synthesized in a two-step procedure that first involved the insertion of the diselenide moiety on a carboxylic acid and was followed by a Steglich esterification reaction between the biscarboxylic acid containing the diselenide unit and 2,6-di(pyridin-2-yl)pyridin-4-ol (tpyOH). The title compound was characterized via FT-IR, Raman, NMR (1D and 2D), and UV-Vis spectroscopies and elemental analysis. Emission properties were investigated.
1. Introduction
2,2′:6′,2″-Terpyridine (tpy) and its derivatives are tridentate ligands widely employed for the synthesis of coordination compounds with several potential applications in catalysis [1,2], medicine [3,4], and supramolecular chemistry [1,5,6,7,8]. A wide variety of publications have focused on the applications of tpy coordination complexes with d- and f-block metals. However, there has been recent interest in the biological properties of the free ligand and its derivatives, which are being tested for their anticancer activity [9,10].
On the other hand, selenium is a micronutrient important to human health, and its deficiency in the human body leads to neurodegenerative diseases, cardiovascular diseases, arthritis, cancer, etc. [11,12]. Diselenides are part of the class of organoselenium compounds and have received increased attention during the last few decades due to their unique chemical and biological properties. These types of compounds have been employed in synthetic transformations with other biologically relevant molecules (e.g., heterocycles), resulting in novel compounds with promising pharmaceutical potential [13,14,15].
A wide variety of tpy-based materials can be prepared by varying the substitution pattern onto the tpy scaffold [2]. Although several tpy derivatives with disulfide moieties are known [16,17,18], to the best of our knowledge, tpy functionalized with a diselenide moiety has never been reported elsewhere. Based on this background, we aimed to synthesize novel tpy derivatives containing a diselenide moiety.
2. Results
2.1. Synthesis
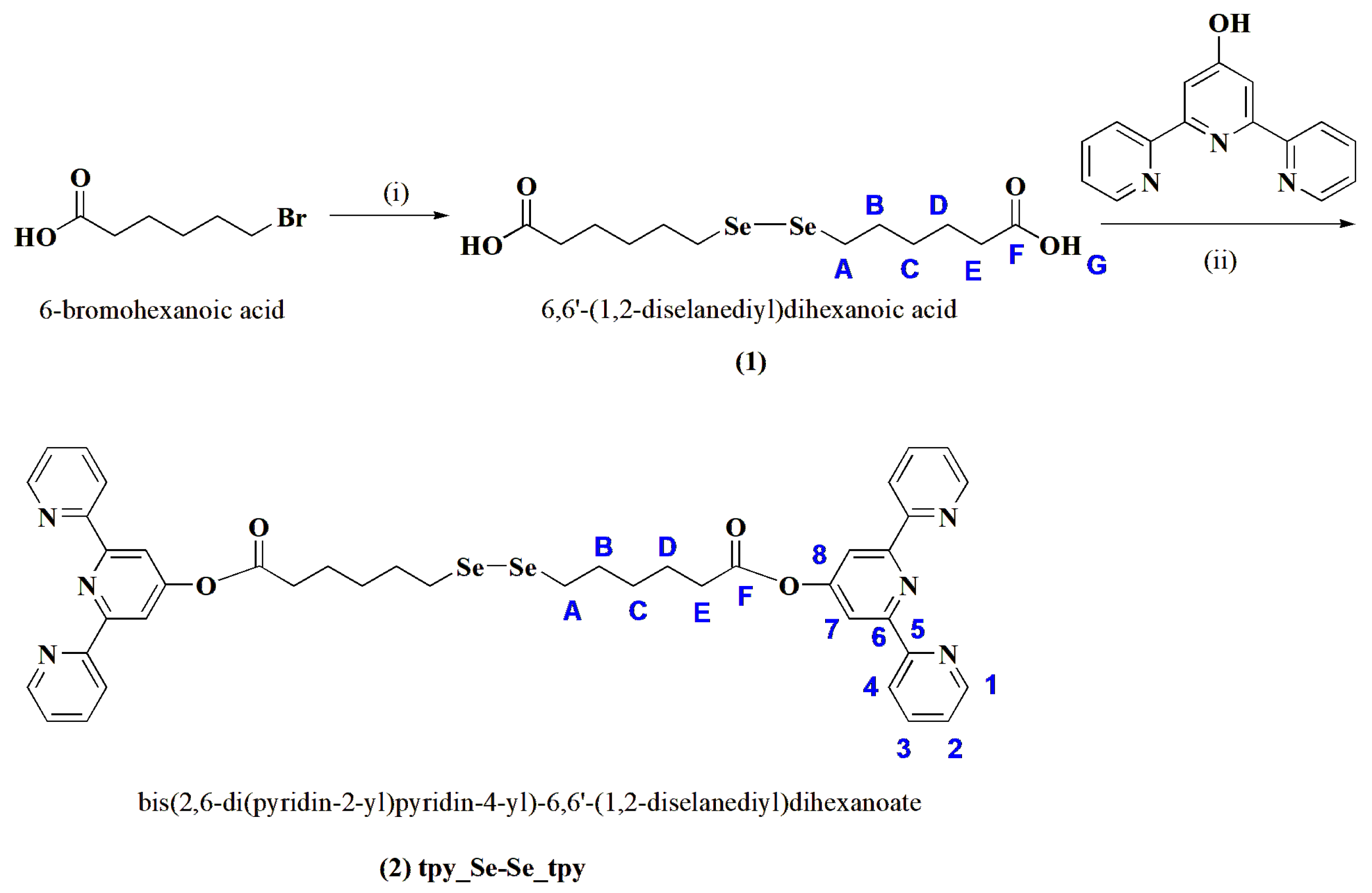
The target compound 2, bis(2,6-di(pyridin-2-yl)pyridin-4-yl)-6,6′-(1,2-diselanediyl)dihexanoate, was obtained in two steps (see Scheme 1). Firstly, 6,6′-(1,2-diselanediyl)dihexanoic acid (compound 1) was synthesized following a method described in the literature [19] via the displacement of bromide from 6-bromohexanoic acid by disodium diselenide, Na2Se2, obtained in situ in an inert atmosphere through the reduction of Se powder by NaBH4. Then, a tetrahydrofuran (THF) solution of 6-bromohexanoic acid was injected, and the reaction mixture was stirred at room temperature until the color changed from dark red to pale yellow. After purification via chromatographic methods, compound 1 was further reacted with 2,6-di(pyridin-2-yl)pyridin-4-ol by a Steglich esterification reaction in the presence of dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP). The pure compound was isolated after two recrystallizations from ethyl acetate, followed by two purifications via column chromatography, first using SiO2 to remove the unreacted compound 1 and the non-polar products of the reaction, and then using neutral Al2O3.
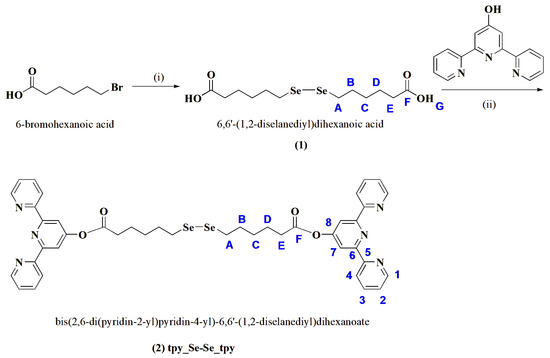
Scheme 1.
Reaction pathway for the synthesis of compounds 1 and 2 and atom labeling (in blue). Reagents and conditions: (i) Se, NaBH4, H2O, 50 °C, then 6-bromohexanoic acid in THF, 24 h at r.t.; (ii) DCC, DMAP, THF, 3 days.
2.2. Characterization
Compounds 1 and 2 were characterized via complementary investigations like spectroscopic methods: FT-IR, Raman, NMR (1D and 2D) and UV-Vis [20,21]. Their purities were confirmed through elemental analysis. Their photophysical properties (emission maxima and yields and lifetimes of the excited states) were determined in a dichloromethane solution.
Although the synthesis of compound 1 has been reported elsewhere [19], only its 1H-NMR and ESI-MS spectra were carried out. Therefore, we have further characterized it through FT-IR, Raman, and NMR (1D and 2D), and its photophysical properties were investigated via UV-Vis and fluorescence studies.
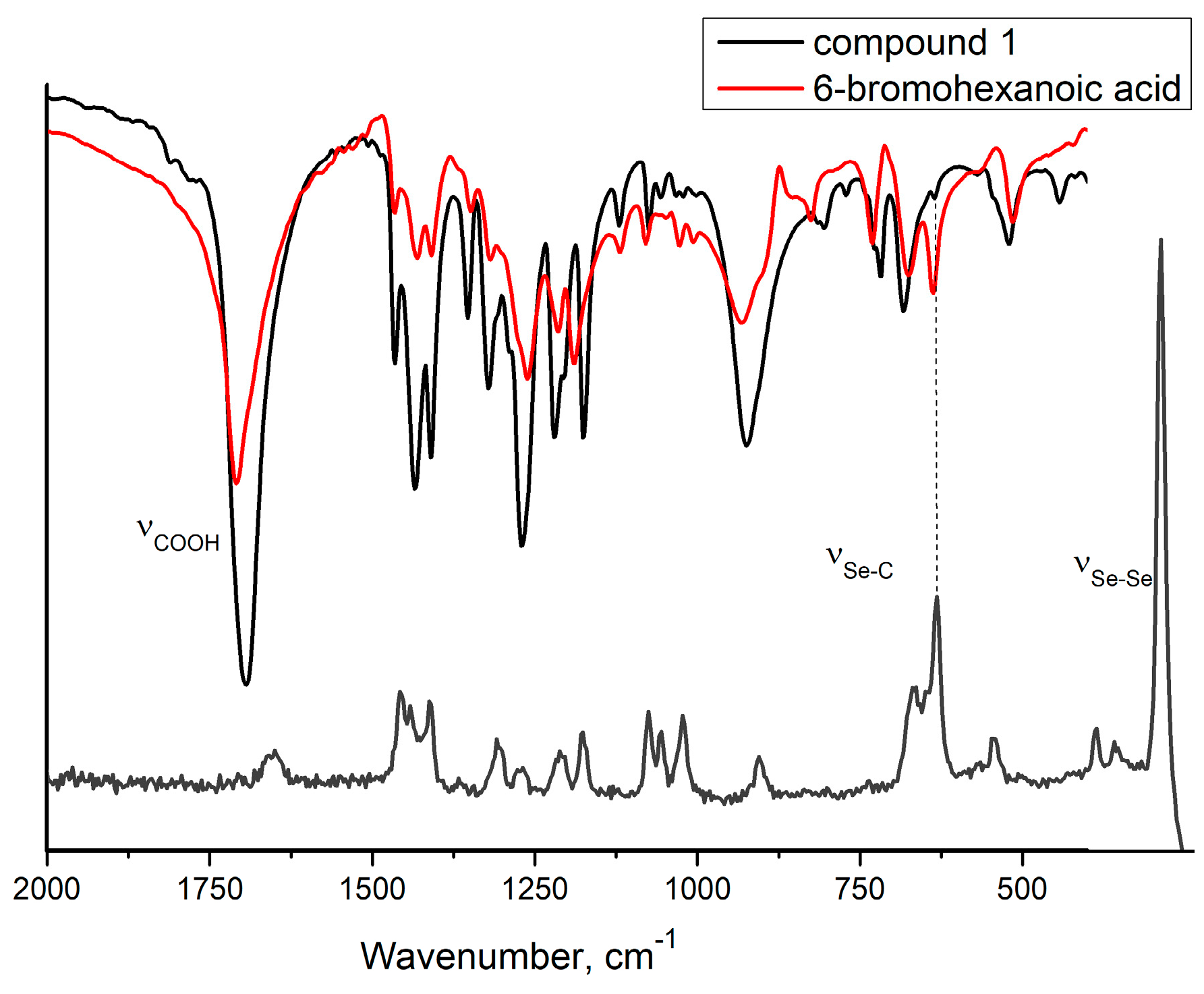
In particular, the first confirmation of the presence of the diselenide group for compound 1 is observed in the vibrational spectra where the stretching vibrations of Se–Se bonds and Se–C bonds are found at 286 cm−1 (Raman) (Figure S7—Supplementary Materials) and 641 cm−1 (Raman and FT-IR) (Figure 1) [22,23]. Moreover, the FT-IR spectrum of compound 1 (Figure S1—Supplementary Materials) presented the characteristic absorption bands for the carboxylic group at 1694 cm−1.
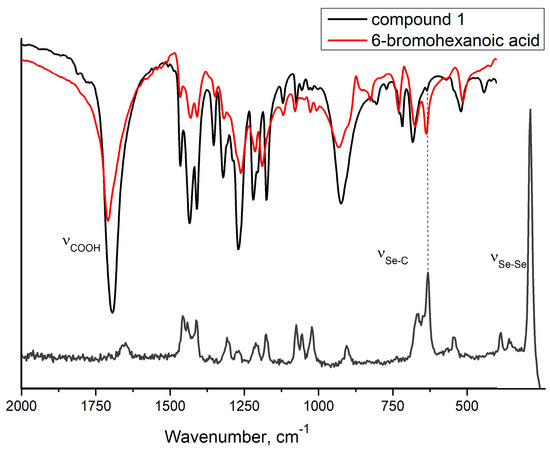
Figure 1.
Region of the FT−IR spectra of compound 1 (black) and 6−bromohexanoic acid (red) and RAMAN spectra of compound 1 (gray).
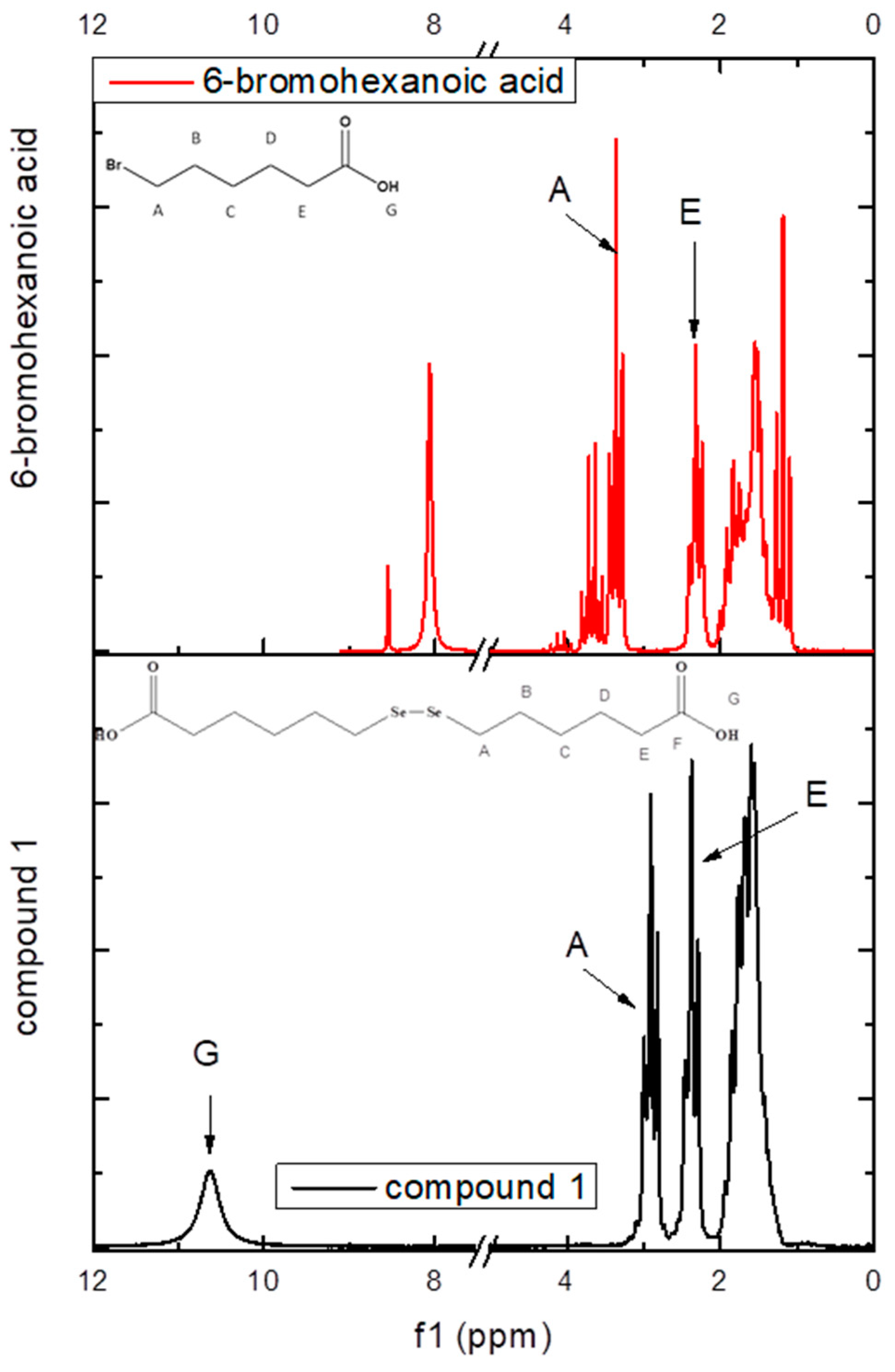
For comparison, the proton spectra of compound 1 and its precursor, 6-bromohexanoic acid, were recorded on an 80 MHz benchtop NMR. As can be seen in Figure 2, the proton HA shifted to a lower frequency from 3.36 ppm for 6-bromohexanoic acid to 2.91 ppm for compound 1, which further confirmed the insertion of the diselenide bond in the molecule.
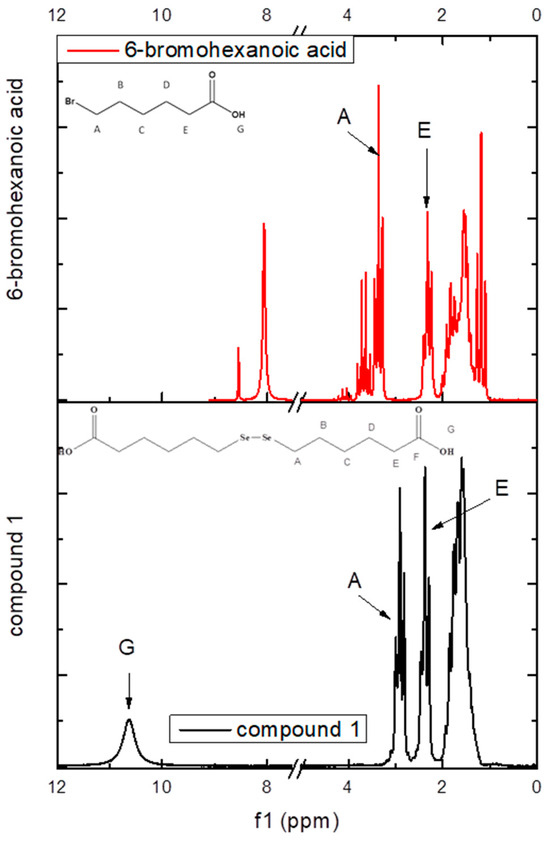
Figure 2.
1H-NMR (80 MHz) spectrum of compound 1 plotted against 6-bromohexanoic acid and their atom labeling.
In order to elucidate the structure, 1D and 2D NMR spectra were recorded on a Bruker 500 MHz instrument (Supplementary Figures S2–S6). All the protons and carbon atoms were attributed based on 1H-1H COSY, HSQC, and HMBC experiments. The data are gathered in Table S1.
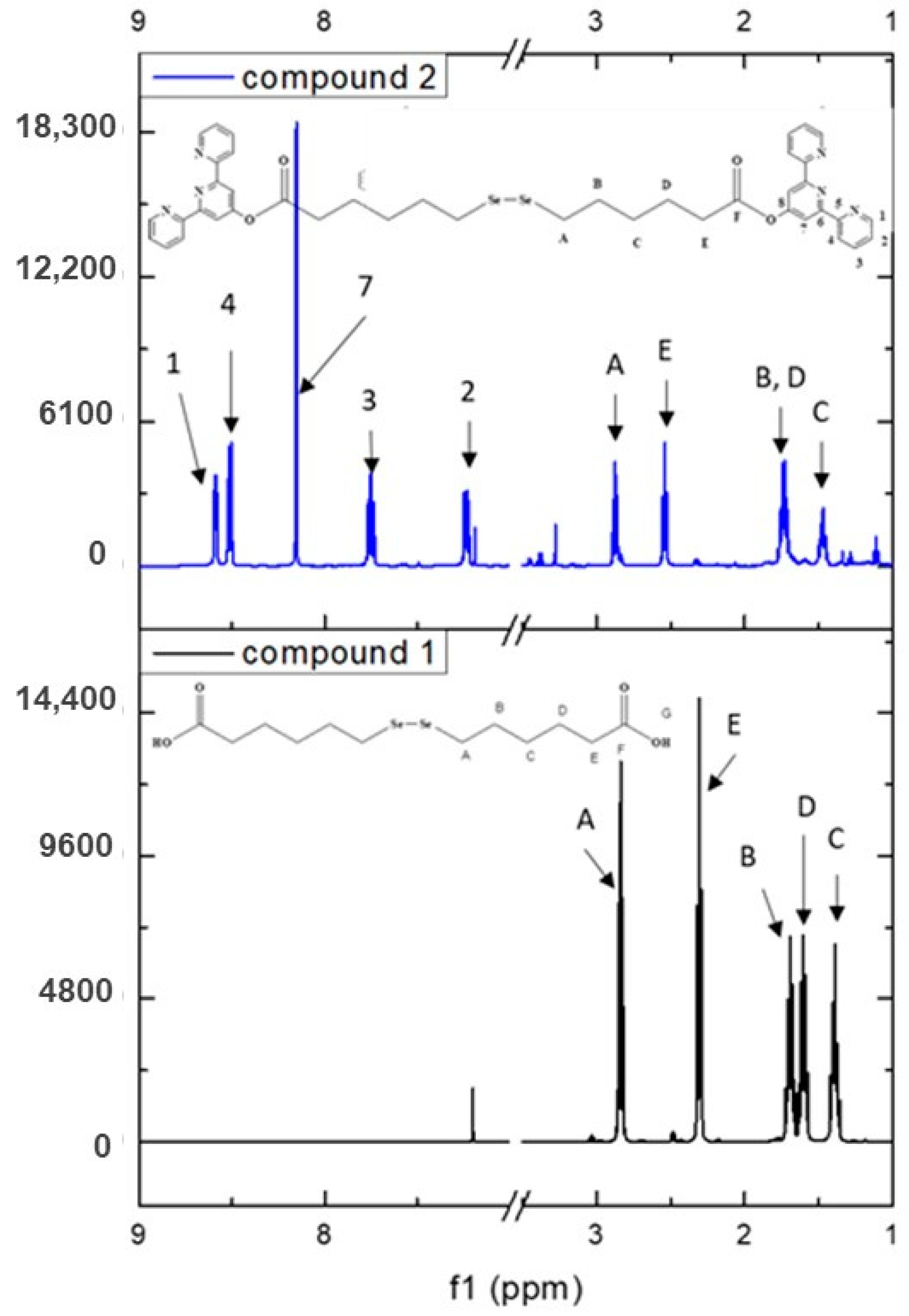
The proton spectrum (Figure 3 and Figure S2—Supplementary Materials) accords well with the NMR results published previously [19].
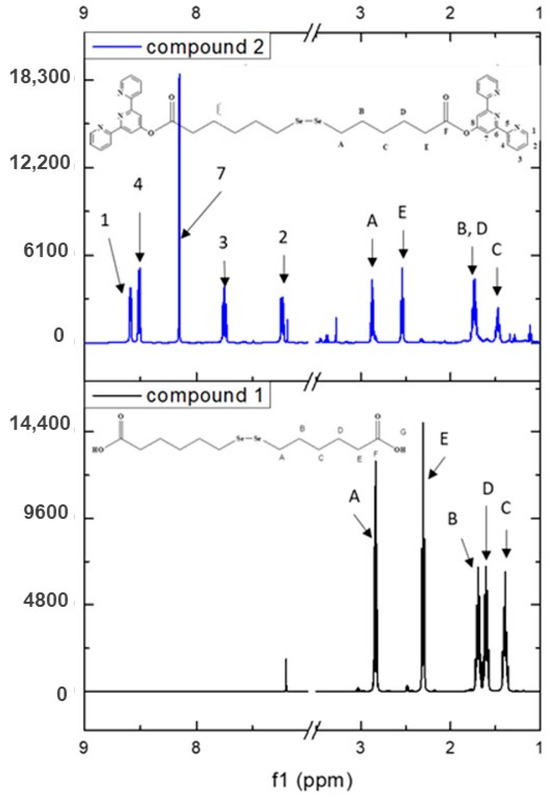
Figure 3.
1H-NMR spectrum of compound 2 plotted against compound 1 and their atom labeling.
A full indexation was also performed for compound 2, where all protons and carbon atoms were attributed based on 1D and 2D NMR (Supplementary Table S2 and Figures S9–S13). The proton spectrum (Figure 4 and Supplementary Figure S9) exhibits the characteristic signals for the tpy moiety supporting the proposed structure. Furthermore, the successful formation of the ester is confirmed via the chemical shift of the proton HE from 2.31 ppm in compound 1 to 2.54 ppm in the tpy derivative (compound 2) (see Figure 3). This is further supported by 13C-NMR (Supplementary Figure S9) where the chemical shift of the carbon labeled G from 180.15 ppm in compound 1 to 170.85 ppm in compound 2 can be observed. Moreover, the 13C-NMR spectra presented the expected signals for all 14 carbon atoms.
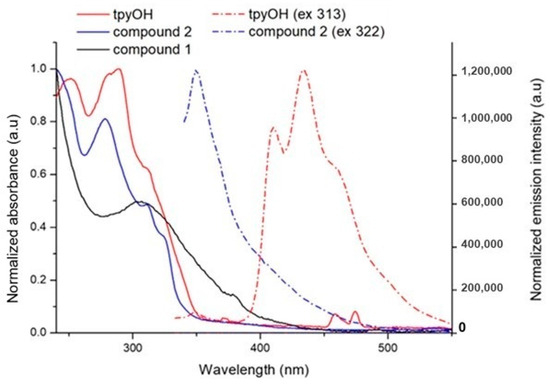
Figure 4.
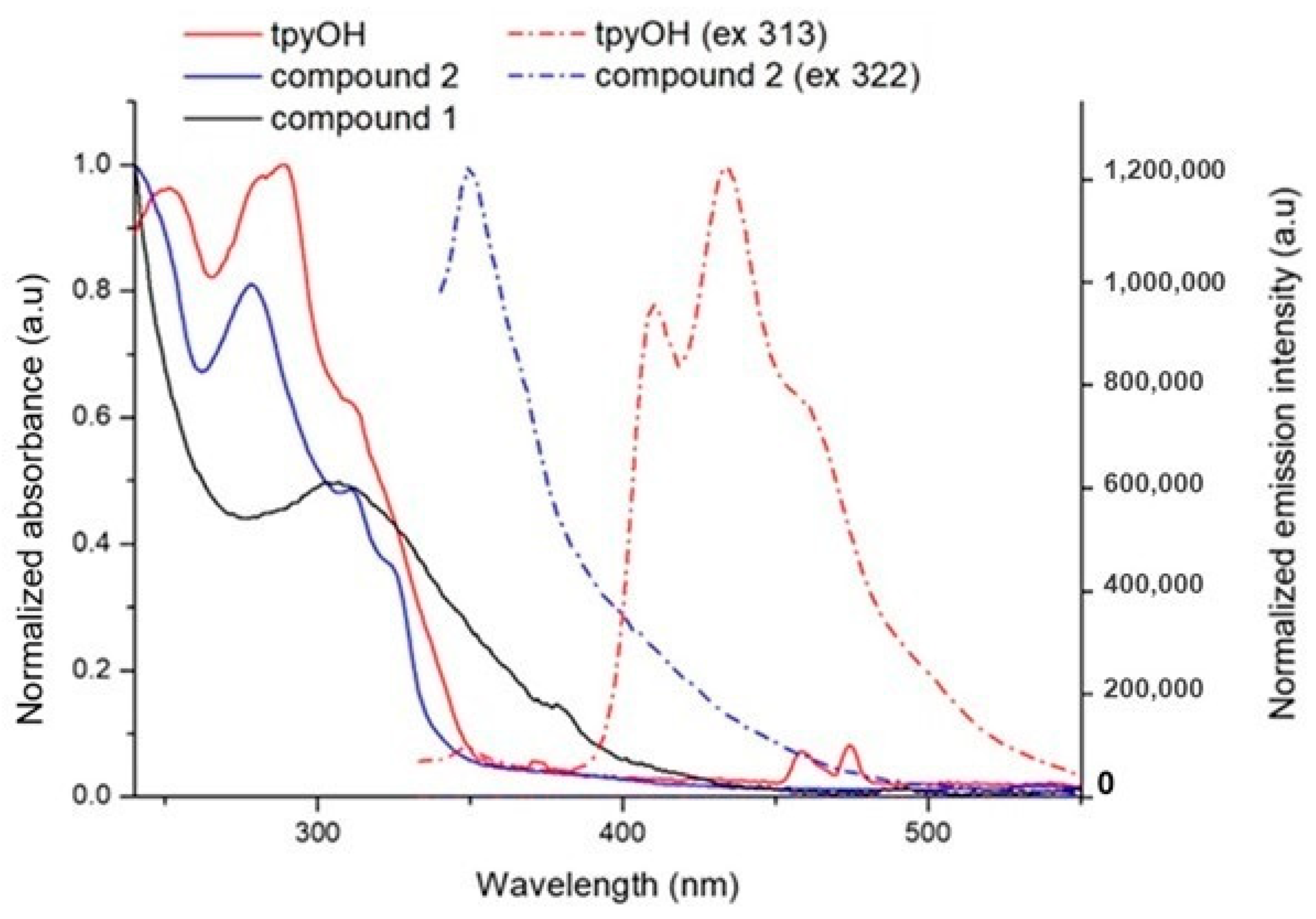
Normalized absorption (straight line) and emission intensity (dashed line) of tpyOH, compound 1 and compound 2 recorded in dichloromethane solutions.
Regarding compound 2, by comparing the FT-IR spectrum of compound 2 with compound 1, it can be seen that the absorption band for the carboxylate appears at 1766 cm−1, which is characteristic of the C=O bond in esters (see Figure S8). Unfortunately, due to the decomposition of compound 2, the Raman spectra could not be registered. However, elemental analysis confirmed the proposed structures of the compounds and their purities.
The photophysical properties of compound 1, compound 2, and tpyOH were collected from freshly prepared dichloromethane solutions, and the data collected are presented in Table 1 and Figure 4.

Table 1.
Photophysical properties in CH2Cl2 solution.
Typical acyclic dialkyl diselenides present an absorption band with a maximum in the range of 286–316 nm, which can be determined by three effects: inductive, hyperconjugative, and torsional (or steric) [24]. Therefore, the absorption band centered at 308 nm in compound 1 was attributed to the diselenide bond.
By comparing the spectra of compound 2 to those of its precursors (tpyOH and compound 1), several pieces of information could be obtained: the absorption band at 290 nm for tpyOH blue shifted to 278 nm for compound 2, with the hyperchromic effect due to n-π* and π-π* from the tpy ring (see Figure 4); the shoulder centered at 322 nm in compound 2 is due to a red shift of the diselenide bond, with compound 2 being more electronegative.
The fluorescence emission was registered for all samples. The data are gathered in Table 1. The emission from the CT excited state for compound 2 decreased compared to the emission for tpyOH, providing an emission quantum yield (ϕ) value of 0.78, and it is accompanied by a bathochromic shift. Time-resolved measurements give a mono-exponential decay of the excited states, with lifetimes between 0.43 and 1.07 ns (Table 1) [5,6].
3. Materials and Methods
2,6-Di(pyridin-2-yl)pyridin-4-ol (tpyOH), selenium powder, sodium borohydride, N,N′-dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (4-DMAP), anhydrous THF, and DMF were purchased from Sigma-Aldrich (St. Luis, MO, USA) and used without further purification. The solvents used for the column chromatography were technical grade and were purchased from Carlo Erba, Emmendingen, Germany.
Infrared spectra (KBr) in the 4000–400 cm−1 range were recorded on a Cary 630 FT-IR spectrophotometer (Agilent, Penang, Malaysia).
Raman spectra of the samples were obtained using the MultiView-2000 system from Nanonics Imaging Ltd., Jerusalem, Israel, equipped with a Shamrock 500i Spectrograph from ANDOR, Belfast, UK. The analysis was performed at room temperature through a 10× microscope objective. The wavelength of laser excitation during the measurements was 514.5 nm with a 30 s exposure time.
The formation of the desired compounds was assessed first on a Fourier 80 MHz benchtop NMR by Bruker. The 1H-NMR and 13C-NMR spectra were recorded on Bruker Avance III 500 MHz (Bruker, Karlsruhe, Germany).
Spectrofluorimetric grade CH2Cl2 was used for photophysical investigations in the solution without further purification. Absorption spectra were recorded using an Agilent Cary 60. A Horiba Fluoromax Plus spectrofluorimeter (HORIBA New Jersey Optical Spectroscopy Center Piscataway, New Jersey, NJ, USA) was used to obtain the emission spectra in the solution using quartz cuvettes of a 1 cm path length. The luminescence quantum yields of the samples in the solution were recorded using a QuantaPhi-2 integrating sphere with 121 mm internal diameter with reflectivity from 250 to 2500 nm. Time-resolved measurements were performed using time-correlated single-photon counting (TCSPC) on the FluoroMax Plus apparatus. NanoLED pulses centered at 370 nm and 461 nm (FWHM 750 ps with 1 MHz repetition rate) were used as the excitation source and fixed directly on the sample chamber at 90° to a single-grating emission monochromator (2.1 nm mm−1 dispersion; 1200 grooves per mm). The data analysis was performed using the commercially available Data Station FluorEssence version 3.9 software (Horiba Jobin Yvon), and EZ time is intended for use with the HORIBA Scientific DeltaDiode and DeltaHub Fluorescence Lifetime systems.
Elemental analyses (CHN) were performed using a Perkin Elmer 2400 microanalyzer.
3.1. Synthesis of Compound 1
In a three-neck round-bottom flask, under argon atmosphere, Se powder (0.202 g, 2.56 mmol) was suspended in ultrapure water (5 mL), and then NaBH4 (0.099 g, 2.64 mmol) was dissolved in ultrapure water (5 mL) and was quickly injected in the reaction mixture. The mixture was then stirred at 70 °C until the color turned dark brown, cooled to room temperature, and 0.500 g (2.56 mmol) of 6-bromohexanoic acid in THF was added dropwise. The reaction mixture was further stirred at room temperature overnight, during which the color turned pale yellow. After the mixture was poured into an extraction funnel and extracted with Et2O (3 × 20 mL), the organic phase was dried over anhydrous sodium sulfate, filtered, and the solvents were evaporated under the reduced pressure. The pure compound was obtained after purification via column chromatography using SiO2 as the stationary phase and CHCl3-MeOH = 95:5 as the eluent, resulting in a yellow solid (1.03 mmol, 40.2%).
FT-IR (KBr, cm−1): 1694 (νC=O, acid), 641 (νC-Se);
Raman: 286 cm−1 (νSe-Se), 641 cm−1 (νC-Se);
1H-NMR (500 MHz, Chloroform-d), δ(ppm): 2.84 (t, J = 7.4 Hz, 4H), 2.31 (t, J = 7.4 Hz, 4H), 1.69 (qu, J = 7.4 Hz, 4H), 1.61 (qu, J = 7.5 Hz, 4H), 1.45–1.34 (m, 4H);
13C-NMR (126 MHz, Chloroform-d), δ(ppm): 180.13, 33.92, 30.56, 29.66, 28.84, 24.13.
3.2. Synthesis of Compound 2
In a three-neck round-bottom flask, under argon atmosphere, compound 1 (0.100 g, 0.26 mmol), 2,6-di(pyridin-2-yl)pyridin-4-ol (tpyOH) (0.129 g, 0.52 mmol) and 4-DMAP (12.71 mg, 0.104 mmol) were dissolved in THF (5 mL) and DMF (1 mL) and were stirred at room temperature for 30 min, and then DCC (0.64 g, 0.312 mmol) was dissolved in THF (3 mL) and was added dropwise. The consumption of the starting materials was monitored on TLC (Al2O3: CHCl3:hexane = 6:4). After 3 days, the solvents from the reaction mixture were evaporated under the reduced pressure, dissolved in CHCl3 and washed with distilled water (3 × 50 mL). The organic phase was dried over anhydrous sodium sulfate, filtered, and the solvents were evaporated under the reduced pressure. The residue was dissolved in AcOEt and the undissolved precipitate was filtered off (to remove the DCU formed) and the mother liquor was evaporated. This procedure was repeated until no precipitate remained when adding AcOEt (3 times). The pure compound was obtained after a process of purification via column chromatography using SiO2 as the stationary phase and CHCl3-MeOH = 95:5 as the eluent and a process of purification via column chromatography using Al2O3 as the stationary phase and CHCl3-hexane = 60:40 as the eluent, resulting in a pale-yellow gel (0.049 mmol, 18.8%).
FT-IR (KBr, cm−1): 1766 (νC=O, ester), 1633–1447 (νC=N and νC=C).
1H-NMR (500 MHz, Chloroform-d), δ(ppm): 8.59 (ddd, J = 4.8, 1.8, 0.9 Hz, 4H), 8.51 (dt, J = 8.0, 1.1 Hz, 4H), 8.15 (s, 4H), 7.75 (td, J = 7.7, 1.8 Hz, 4H), 7.25–7.18 (m, 4H), 2.87 (t, J = 7.4 Hz, 4H), 2.54 (t, J = 7.5 Hz, 4H), 1.73 (dt, J = 15.3, 7.6 Hz, 8H), 1.51–1.43 (m, 4H).
13C-NMR (126 MHz, Chloroform-d), δ(ppm): 170.85, 159.72, 157.56, 155.33, 149.14, 136.90, 124.12, 121.30, 114.28, 34.26, 30.62, 29.59, 28.91, 24.32.
Anal. calcd. for C42H40N6O4Se2 (M = 850.72 g/mol): C, 59.30; H, 4.74; N, 9.88; found: C, 59.76; H, 4.73; N, 10.08.
4. Conclusions
This paper reports the synthesis and characterization of a tpy derivative containing a diselenide unit. Its structure was elucidated based on FT-IR, Raman, and NMR (1D and 2D) investigations, while its optical properties have been studied via UV-Vis and fluorescence measurements.
The target compound 2 was obtained in two steps, which involved the obtainment of compound 1 via the displacement of bromide from 6-bromohexanoic acid by disodium diselenide, followed by a Steglich esterification reaction.
The first confirmation of the presence of the diselenide group was observed in the vibrational spectra of compound 1 and was further supported by the chemical shift of the proton neighboring the Se atom. Further, the chemical shift of the proton neighboring the carboxylic group confirmed the formation of compound 2.
These compounds are of interest for the further functionalization of silica or noble nanoparticles. Among these, gold nanoparticles functionalized with terpyridine bearing a diselenide unit can be applied in biomedicine for drug loading or as drug carriers. Moreover, diselenides are known to stabilize gold nanoparticles better than other types of commonly employed linkages, such as disulfide.
Supplementary Materials
Figure S1: FT-IR spectrum of compound 1; Figure S2: 1H-NMR spectrum of compound 1 recorded in CDCl3; Figure S3: 13C-NMR spectrum of compound 1 recorded in CDCl3; Figure S4: 1H-1H (COSY) spectrum of compound 1 recorded in CDCl3; Figure S5: HSQC spectrum of compound 1 recorded in CDCl3; Figure S6: HMBC spectrum of compound 1 recorded in CDCl3; Table S1: 1D and 2D NMR of compound 1 recorded in CDCl3; Figure S7: Raman spectrum of compound 1; Figure S8: FT-IR spectrum of compound 2 plotted against compound 1; Figure S9: 1H-NMR spectrum of compound 2 recorded in CDCl3; Figure S10: 13C-NMR spectrum of compound 2 recorded in CDCl3; Figure S11: 1H-1H (COSY) spectrum of compound 2; Figure S12: HSQC spectrum of compound 2; Figure S13: HMBC spectrum of compound 2; and Table S2: 1D and 2D NMR assignment of compound 2 recorded in CDCl3.
Author Contributions
Design of the experiments, A.A.A. and E.I.S.; performance of the experiments, A.A.A.; analysis of the spectral data, E.P., V.B., P.S. and A.A.A.; writing the manuscript, A.A.A.; supervision and funding acquisitions, A.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Ministry of Research, Innovation and Digitization, CNCS/CCCDI—UEFISCDI, project number PN-III-P1-1.1-PD-2021-0427, within PNCDI III.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
This work was supported by a grant from the Ministry of Research, Innovation and Digitization, CNCS/CCCDI—UEFISCDI, project number PN-III-P1-1.1-PD-2021-0427, within PNCDI III. The authors acknowledge the Romanian Academy, Program 4 and the infrastructure support granted by the project RO-OPENSCREEN, MySMIS code: 127952, Contract no. 371/20.07.2020, co-financed by the European Regional Development Fund through the Competitiveness Operational Program 2014–2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wei, C.; He, Y.; Shi, X.; Song, Z. Terpyridine-metal complexes: Applications in catalysis and supramolecular chemistry. Coord. Chem. Rev. 2019, 35, 1–19. [Google Scholar] [CrossRef]
- Husson, J. 4′-(N-(2-Cyanoethyl)pyrrol-2-yl)-2,2′:6′,2″-terpyridine. Molbank 2023, 2023, M1689. [Google Scholar] [CrossRef]
- Eryazici, I.; Moorefield, C.N.; Newkome, G.R. Square-Planar Pd(II), Pt(II), and Au(III) Terpyridine Complexes: Their Syntheses, Physical Properties, Supramolecular Constructs, and Biomedical Activities. Chem. Rev. 2008, 108, 1834–1895. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Gottschaldt, M.; Newkome, G.R.; Schubert, U.S. Terpyridines and their Complexes with First Row Transition Metal Ions: Cytotoxicity, Nuclease Activity and Self-Assembly of Biomacromolecules. Curr. Top. Med. Chem. 2012, 12, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Andelescu, A.A.; Heinrich, B.; Spirache, M.A.; Voirin, E.; La Deda, M.; Di Maio, G.; Szerb, E.I.; Donnio, B.; Costisor, O. Playing with Pt (II) and Zn (II) coordination to obtain luminescent metallomesogens. Chem. Eur. J. 2020, 26, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- La Deda, M.; Di Maio, G.; Candreva, A.; Heinrich, B.; Andelescu, A.-A.; Popa, E.; Voirin, E.; Badea, V.; Amati, M.; Costişor, O.; et al. Very intense polarized emission in self-assembled room temperature metallomesogens based on Zn(II) coordination complexes: An experimental and computational study. J. Mater. Chem. C 2022, 10, 115–125. [Google Scholar] [CrossRef]
- Andelescu, A.A.; Ilies, S.; Cretu, C.; Popa, E.; Marinescu, S.; Heinrich, B.; Manea, F.; Negrea, S.; Donnio, B.; Szerb, E.I. Pentacoordinated Liquid Crystalline Zn (II) Complex Organized in Smectic Mesophase: Synthesis, Structural and Electrochemical Properties. Appl. Sci. 2022, 12, 8306. [Google Scholar] [CrossRef]
- Popa, E.; Andelescu, A.A.; Ilies, S.; Visan, A.; Cretu, C.; Scarpelli, F.; Crispini, A.; Manea, F.; Szerb, E.I. Hetero-Bimetallic Ferrocene-Containing Zinc(II)-Terpyridyl-Based Metallomesogen: Structural and Electrochemical Characterization. Materials 2023, 16, 1946. [Google Scholar] [CrossRef]
- Malarz, K.; Zych, D.; Gawecki, R.; Kuczak, M.; Musioł, R.; Mrozek-Wilczkiewicz, A. New derivatives of 4’-phenyl-2,2’:6’,2’’-terpyridine as promising anticancer agents. Eur. J. Med. Chem. 2021, 212, 113032. [Google Scholar] [CrossRef]
- Musiol, R.; Malecki, P.; Pacholczyk, M.; Mularski, J. Terpyridines as promising antitumor agents: An overview of their discovery and development. Expert Opin. Drug. Discov. 2022, 17, 259–271. [Google Scholar] [CrossRef]
- Kunwar, A.; Bansal, P.; Jaya Kumar, S.; Bag, P.P.; Paul, P.; Reddy, N.D.; Kumbhare, L.B.; Jain, V.K.; Chaubey, R.C.; Unnikrishnan, M.K.; et al. In vivo radioprotection studies of 3,3′-diselenodipropionic acid, a selenocystine derivative. Free Radic. Biol. Med. 2020, 48, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Zhong, M.; Li, S.; Li, X.; Zhang, Y.; Zhang, Y.; He, X. Synthesis and potential anti-cancer activity of some novel 3 selenocyanates and diselenides. Chem. Biodivers. 2020, 17, e1900603. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.D.; Divac, V.M. Diselenides and Selenocyanates as Versatile Precursors for the Synthesis of Pharmaceutically Relevant Compounds. Curr. Org. Synth. 2022, 19, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.; Koketsu, M. Recent developments in the synthesis of biologically relevant selenium-containing scaffolds. Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef] [PubMed]
- Trang, C.D.M.; Saala, T.; Inkpen, M.S. Methyldisulfide groups enable the direct connection of air-stable metal bis(terpyridine) complexes to gold surfaces. Dalton Trans. 2023, 52, 7836–7842. [Google Scholar] [CrossRef]
- Constable, E.C.; Hermann, B.A.; Housecroft, C.E.; Neuburger, M.; Schaffner, S.; Scherer, L.J. 2,2′:6′,2″-Terpyridine-4′(1′H)-thione: A missing link in metallosupramolecular chemistry. N. J. Chem. 2005, 29, 1475–1481. [Google Scholar] [CrossRef]
- Romashkina, R.B.; Majouga, A.G.; Beloglazkina, E.K.; Pichugina, D.A.; Askerka, M.S.; Moiseeva, A.A.; Rakhimov, R.D.; Zyk, N.V. Sulfur-containing terpyridine derivatives: Synthesis, coordination properties, and adsorption on the gold surface. Russ. Chem. Bull. 2012, 61, 2265–2281. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, W.; Pei, Q.; Hu, X.; Xie, Z.; Jing, X. Redox-Hypersensitive Organic Nanoparticles for Selective Treatment of Cancer Cells. Chem. Mater. 2016, 28, 4440–4446. [Google Scholar] [CrossRef]
- Han, W.; Zhang, S.; Qian, J.; Zhang, J.; Wang, X.; Xie, Z.; Xu, B.; Han, Y.; Tian, W. Redox Responsive Fluorescent nanoparticles based on Diselenide-containing AIEgens for Cell imaging and Selective Cancer Therapy. Chem. Asian J. 2019, 14, 1745–1753. [Google Scholar] [CrossRef]
- Frizon, T.E.; Rafique, J.; Saba, S.; Bechtold, I.H.; Gallardo, H.; Braga, A.L. Synthesis of Functionalized Organoselenium Materials: Selenides and Diselenides Containing Cholesterol. EurJOC 2015, 16, 3470–3476. [Google Scholar] [CrossRef]
- Hailemeskel, B.Z.; Addisu, K.D.; Prasannan, A.; Lakew, M.S.; Kao, C.-Y.; Tsai, H.-C. Synthesis and characterization of diselenide linked poly(ethylene glycol) nanogel as multi-responsive drug carrier. Appl. Surf. Sci. 2018, 449, 15–22. [Google Scholar] [CrossRef]
- Helios, K.; Pietraszko, A.; Zierkiewicz, W.; Wójtowicz, H.; Michalska, D. The crystal structure, infrared, Raman and density functional studies of bis(2-aminophenyl) diselenide. Polyhedron 2011, 30, 2466–2472. [Google Scholar] [CrossRef]
- Back, T.G.; Codding, P.W. Studies of the dihedral angle of a crowded diselenide by X-ray crystallography and ultraviolet spectroscopy. Can. J. Chem. 1983, 61, 2749–2752. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).