2-(4-(Fluorosulfonyloxy)phenyl)benzoxazole
Abstract
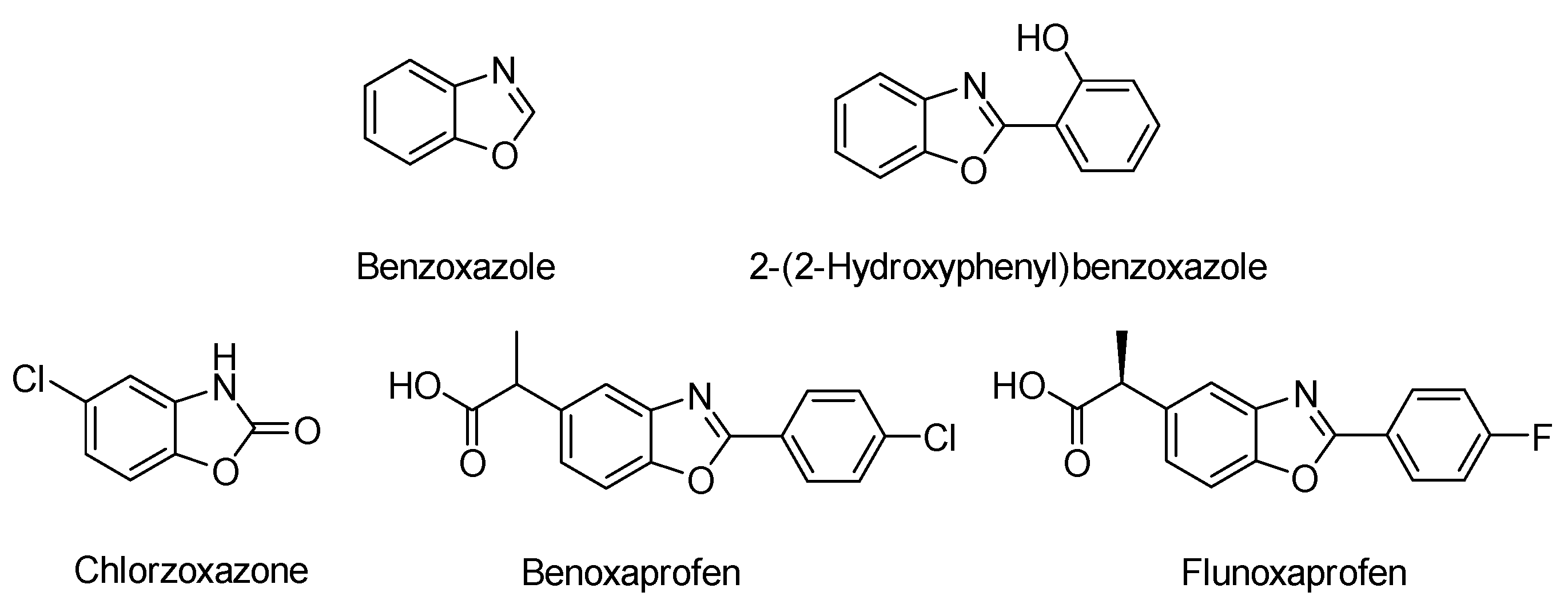
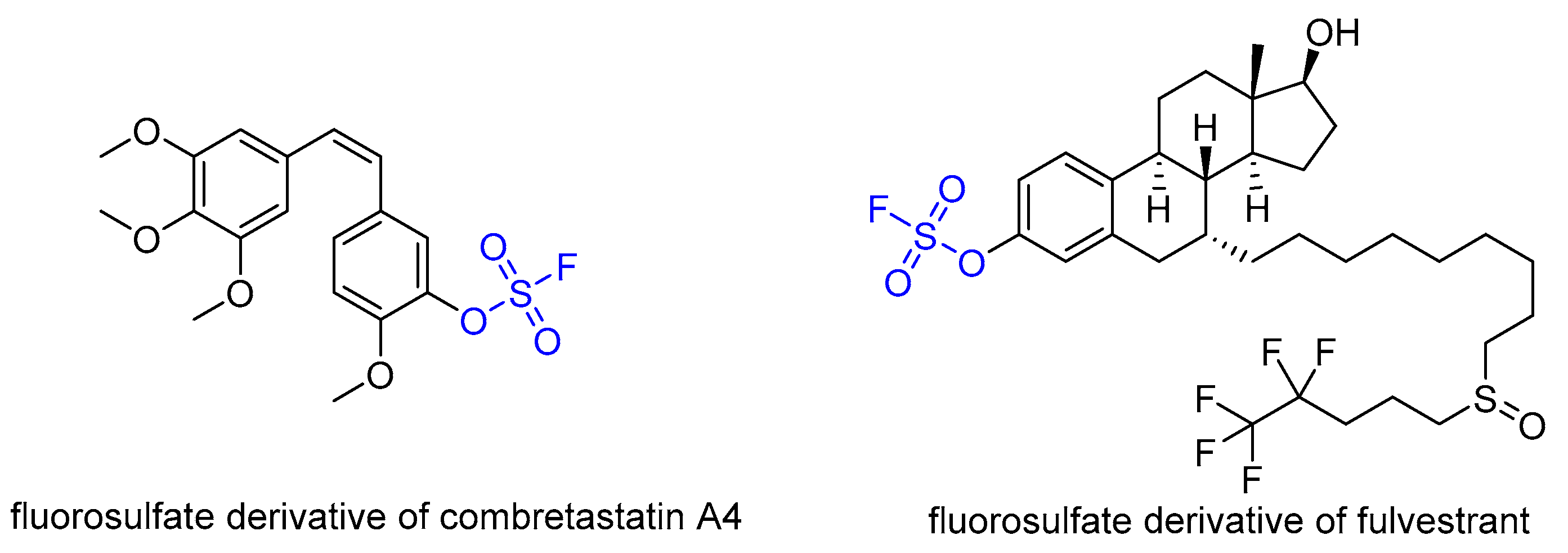
:1. Introduction
2. Results and Discussion
Fluorescent Properties
3. Materials and Methods
3.1. General Information and Compounds Synthesis
3.2. Fluoresent Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.; Veeraswamy, G.; Bhattarai, D.; Goo, J.-I.; Lee, K.; Choi, Y. Recent Advances in the Development of Pharmacologically Active Compounds That Contain a Benzoxazole Scaffold. Asian J. Org. Chem. 2015, 4, 1338–1361. [Google Scholar] [CrossRef]
- Murty, M.S.R.; Ram, K.R.; Rao, R.V.; Yadav, J.S.; Rao, J.V.; Cheriyan, V.T.; Anto, R.J. Synthesis and Preliminary Evaluation of 2-Substituted-1,3-Benzoxazole and 3-[(3-Substituted)Propyl]-1,3-Benzoxazol-2(3H)-One Derivatives as Potent Anticancer Agents. Med. Chem. Res. 2011, 20, 576–586. [Google Scholar] [CrossRef]
- Saluja, P.; Sharma, H.; Kaur, N.; Singh, N.; Jang, D.O. Benzimidazole-Based Imine-Linked Chemosensor: Chromogenic Sensor for Mg2+ and Fluorescent Sensor for Cr3+. Tetrahedron 2012, 68, 2289–2293. [Google Scholar] [CrossRef]
- Tanaka, K.; Kumagai, T.; Aoki, H.; Deguchi, M.; Iwata, S. Application of 2-(3,5,6-Trifluoro-2-Hydroxy-4-Methoxyphenyl)Benzoxazole and -Benzothiazole to Fluorescent Probes Sensing pH and Metal Cations. J. Org. Chem. 2001, 66, 7328–7333. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Krasnova, L.; Finn, M.G.; Sharpless, K.B. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chemie Int. Ed. 2014, 53, 9430–9448. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.S.; Smedley, C.J.; Zheng, Q.; Li, S.; Dong, J.; Moses, J.E. The Growing Applications of SuFEx Click Chemistry. Chem. Soc. Rev. 2019, 48, 4731–4758. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Li, S.; Li, G.; Sharpless, K.B.; Wu, P. SuFEx Click Chemistry Enabled Late-Stage Drug Functionalization. J. Am. Chem. Soc. 2018, 140, 2919–2925. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, N.; Shmalyuk, V.; Khlebnikov, A. 2-(2-(Fluorosulfonyloxy)Phenyl)Benzoxazole. Molbank 2021, 2021, M1242. [Google Scholar] [CrossRef]
- Liang, D.; Streefkerk, D.E.; Jordaan, D.; Wagemakers, J.; Baggerman, J.; Zuilhof, H. Silicon-Free SuFEx Reactions of Sulfonimidoyl Fluorides: Scope, Enantioselectivity, and Mechanism. Angew. Chem. 2020, 132, 7564–7570. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Jacquemin, D.; Perpète, E.A.; Ciofini, I.; Adamo, C.; Valero, R.; Zhao, Y.; Truhlar, D.G. On the Performances of the M06 Family of Density Functionals for Electronic Excitation Energies. J. Chem. Theory Comput. 2010, 6, 2071–2085. [Google Scholar] [CrossRef] [PubMed]
- Ertan, T.; Yildiz, I.; Tekiner-Gulbas, B.; Bolelli, K.; Temiz-Arpaci, O.; Ozkan, S.; Kaynak, F.; Yalcin, I.; Aki, E. Synthesis, Biological Evaluation and 2D-QSAR Analysis of Benzoxazoles as Antimicrobial Agents. Eur. J. Med. Chem. 2009, 44, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Papajak, E.; Zheng, J.; Xu, X.; Leverentz, H.R.; Truhlar, D.G. Perspectives on Basis Sets Beautiful: Seasonal Plantings of Diffuse Basis Functions. J. Chem. Theory Comput. 2011, 7, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 vers. A03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
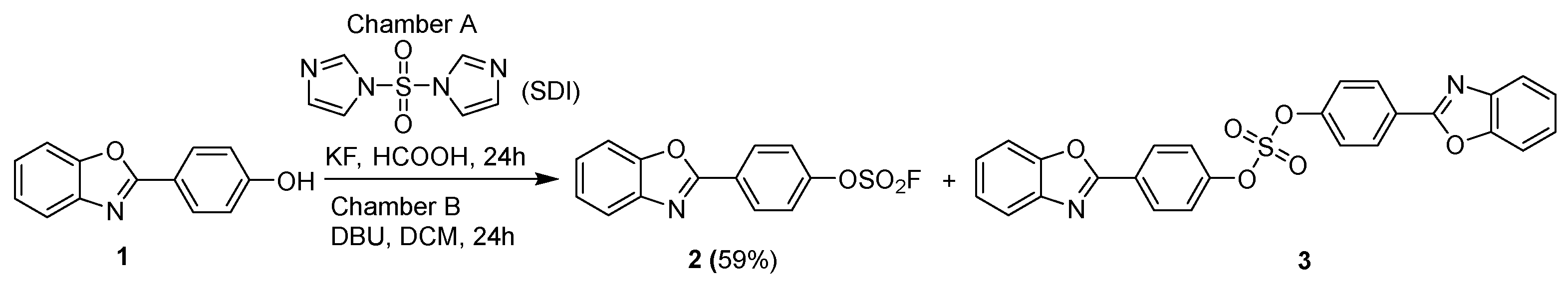
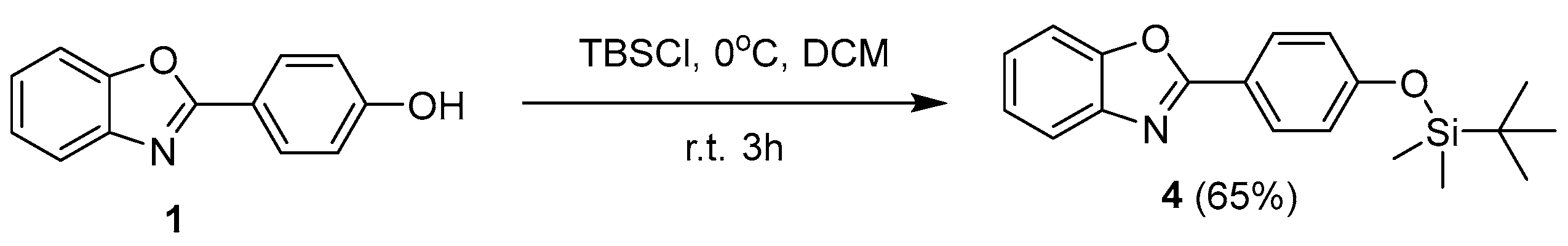

| No. * | Chamber B | Target Product Yield, % ** | |||
|---|---|---|---|---|---|
| Substrate | mmol | DBU, mmol | DCM, mL | ||
| 1 | 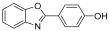 | 0.5 | 1 | 3 | 59 |
| 2 | 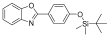 | 0.5 | 1 | 3 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danilenko, N.V.; Lutsuk, M.O.; Patlasova, S.E.; Korotkova, E.I.; Khlebnikov, A.I. 2-(4-(Fluorosulfonyloxy)phenyl)benzoxazole. Molbank 2023, 2023, M1746. https://doi.org/10.3390/M1746
Danilenko NV, Lutsuk MO, Patlasova SE, Korotkova EI, Khlebnikov AI. 2-(4-(Fluorosulfonyloxy)phenyl)benzoxazole. Molbank. 2023; 2023(4):M1746. https://doi.org/10.3390/M1746
Chicago/Turabian StyleDanilenko, Nadezhda V., Mariia O. Lutsuk, Svetlana E. Patlasova, Elena I. Korotkova, and Andrei I. Khlebnikov. 2023. "2-(4-(Fluorosulfonyloxy)phenyl)benzoxazole" Molbank 2023, no. 4: M1746. https://doi.org/10.3390/M1746
APA StyleDanilenko, N. V., Lutsuk, M. O., Patlasova, S. E., Korotkova, E. I., & Khlebnikov, A. I. (2023). 2-(4-(Fluorosulfonyloxy)phenyl)benzoxazole. Molbank, 2023(4), M1746. https://doi.org/10.3390/M1746







