1-(2,5-Dimethoxy-4-nitrophenyl)piperidine
Abstract
:1. Introduction
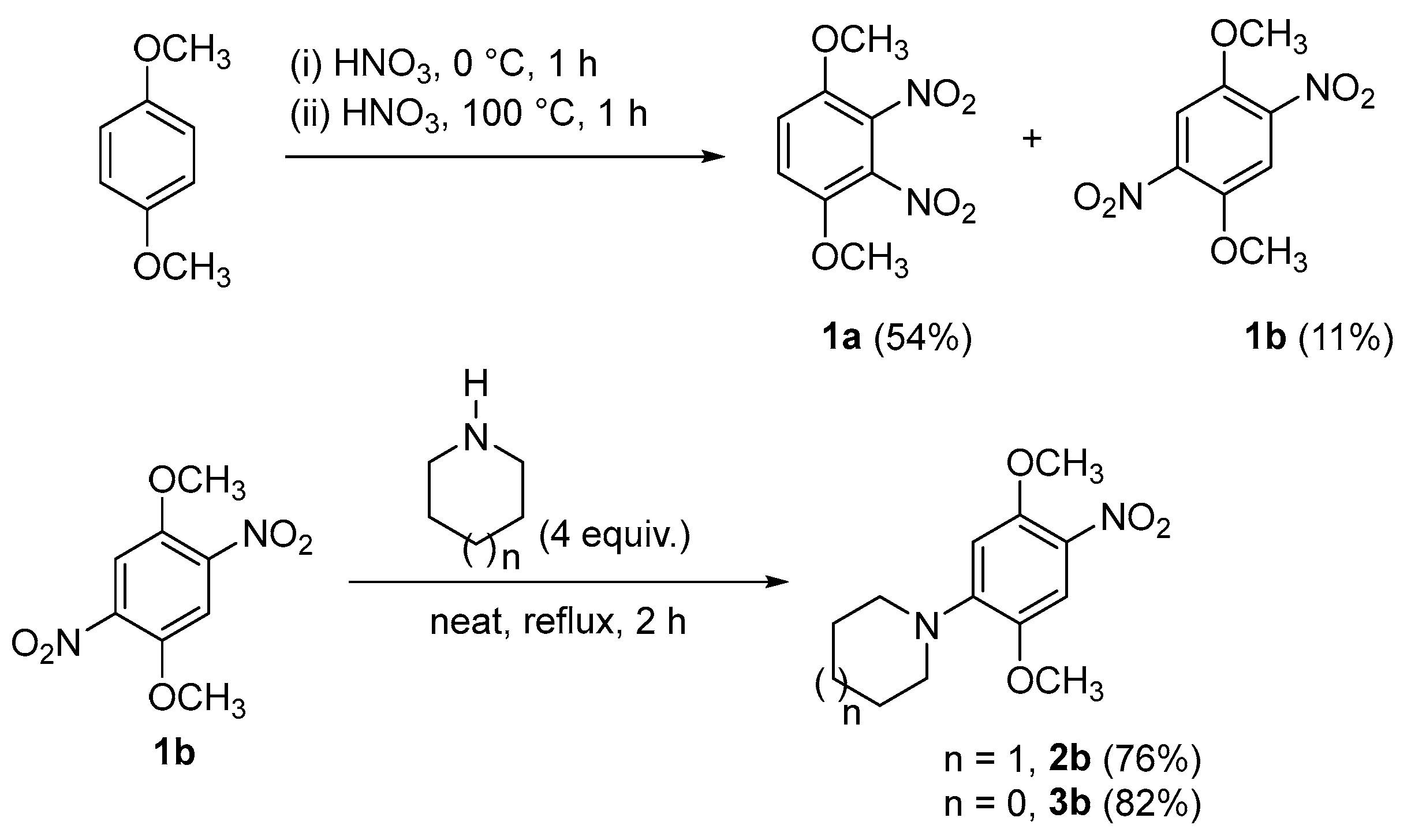
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Measurements
3.2. Synthesis of 1-(2,5-Dimethoxy-4-nitrophenyl)piperidine (2b)
3.3. Synthesis of 1-(2,5-Dimethoxy-4-nitrophenyl)pyrrolidine (3b)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sweeney, M.; Conboy, D.; Mirallai, S.I.; Aldabbagh, F. Advances in the synthesis of ring-fused benzimidazoles and imidazobenzimidazoles. Molecules 2021, 26, 2684. [Google Scholar] [CrossRef] [PubMed]
- Gellis, A.; Kovacic, H.; Boufatah, N.; Vanelle, P. Synthesis and cytotoxicity evaluation of some benzimidazole-4,7-diones as bioreductive anticancer agents. Eur. J. Med. Chem. 2008, 43, 1858–1864. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, L.; Carty, M.P.; Aldabbagh, F. First synthesis of N-[(aziridin-2-yl)methyl]benzimidazolequinone and analysis of toxicity towards normal and Fanconi anemia cells. Chem. Commun. 2008, 43, 5592–5594. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Hehir, S.; Kavanagh, P.; Leech, D.; O’Shaughnessy, J.; Carty, M.P.; Aldabbagh, F. Synthesis by radical cyclization and cytotoxicity of highly potent bioreductive alicyclic ring fused [1,2-a]benzimidazolequinones. Chem. Eur. J. 2007, 13, 3218–3226. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, E.; Carr, M.; Bonham, S.; Carty, M.P.; Aldabbagh, F. Synthesis and toxicity towards normal and cancer cell lines of benzimidazolequinones containing fused aromatic rings and 2-aromatic ring substituents. Eur. J. Med. Chem. 2010, 45, 3762–3769. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.; Gurry, M.; Keane, L.-A.J.; Aldabbagh, F. Greener synthesis using hydrogen peroxide in ethyl acetate of alicyclic ring-fused benzimidazoles and anti-tumour benzimidazolequinones. Tetrahedron Lett. 2017, 58, 3565–3567. [Google Scholar] [CrossRef]
- Shopsowitz, K.; Lelj, J.; MacLachlan, M.J. Regioselectivity in the nitration of dialkoxybenzenes. J. Org. Chem. 2011, 76, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Hammershøj, P.; Reenberg, T.K.; Pittelkow, M.; Nielsen, C.B.; Hammerich, O.; Christensen, J.B. Synthesis and properties of 2,3-dialkynyl-1,4-benzoquinones. Eur. J. Org. Chem. 2006, 2006, 2786–2794. [Google Scholar] [CrossRef]
- CAS Registry Number: 71230-77-8, Catalogue Number: CA0784117. Chemieliva Pharmaceutical, Product List, Jiang Bei Chongqing, China. Available online: https://www.chemieliva.com/new_content.html?casno=71230-77-8 (accessed on 16 October 2023).
- CAS Registry Number: 71230-77-8, Catalogue Number: PR-518937. Atomax Chemicals, Product List, Shenzhen, Guangdong, China. Available online: http://en.atomaxchem.com/71230-77-8.html (accessed on 16 October 2023).
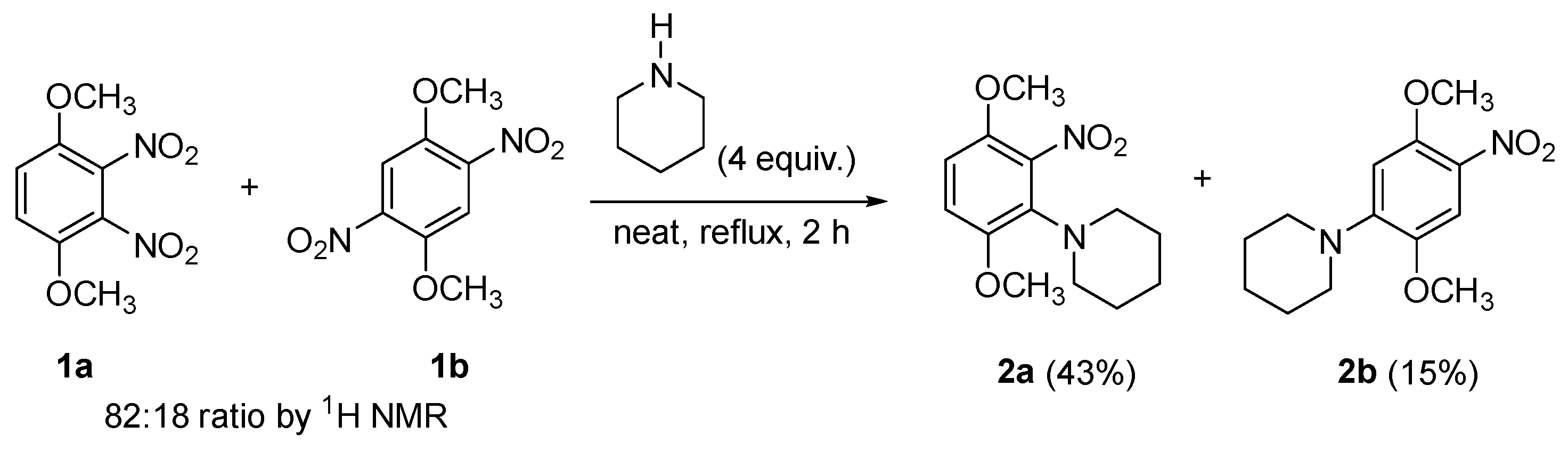

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syeda, T.M.; Al-Shaidi, M.R.; Basri, I.; Merzouk, M.; Aldabbagh, F. 1-(2,5-Dimethoxy-4-nitrophenyl)piperidine. Molbank 2023, 2023, M1744. https://doi.org/10.3390/M1744
Syeda TM, Al-Shaidi MR, Basri I, Merzouk M, Aldabbagh F. 1-(2,5-Dimethoxy-4-nitrophenyl)piperidine. Molbank. 2023; 2023(4):M1744. https://doi.org/10.3390/M1744
Chicago/Turabian StyleSyeda, Tanjia M., Muadh R. Al-Shaidi, Ibtihal Basri, Mahboub Merzouk, and Fawaz Aldabbagh. 2023. "1-(2,5-Dimethoxy-4-nitrophenyl)piperidine" Molbank 2023, no. 4: M1744. https://doi.org/10.3390/M1744
APA StyleSyeda, T. M., Al-Shaidi, M. R., Basri, I., Merzouk, M., & Aldabbagh, F. (2023). 1-(2,5-Dimethoxy-4-nitrophenyl)piperidine. Molbank, 2023(4), M1744. https://doi.org/10.3390/M1744









