tert-Butyl N-Hydroxycarbamate (N-Boc-Hydroxylamine)
Abstract
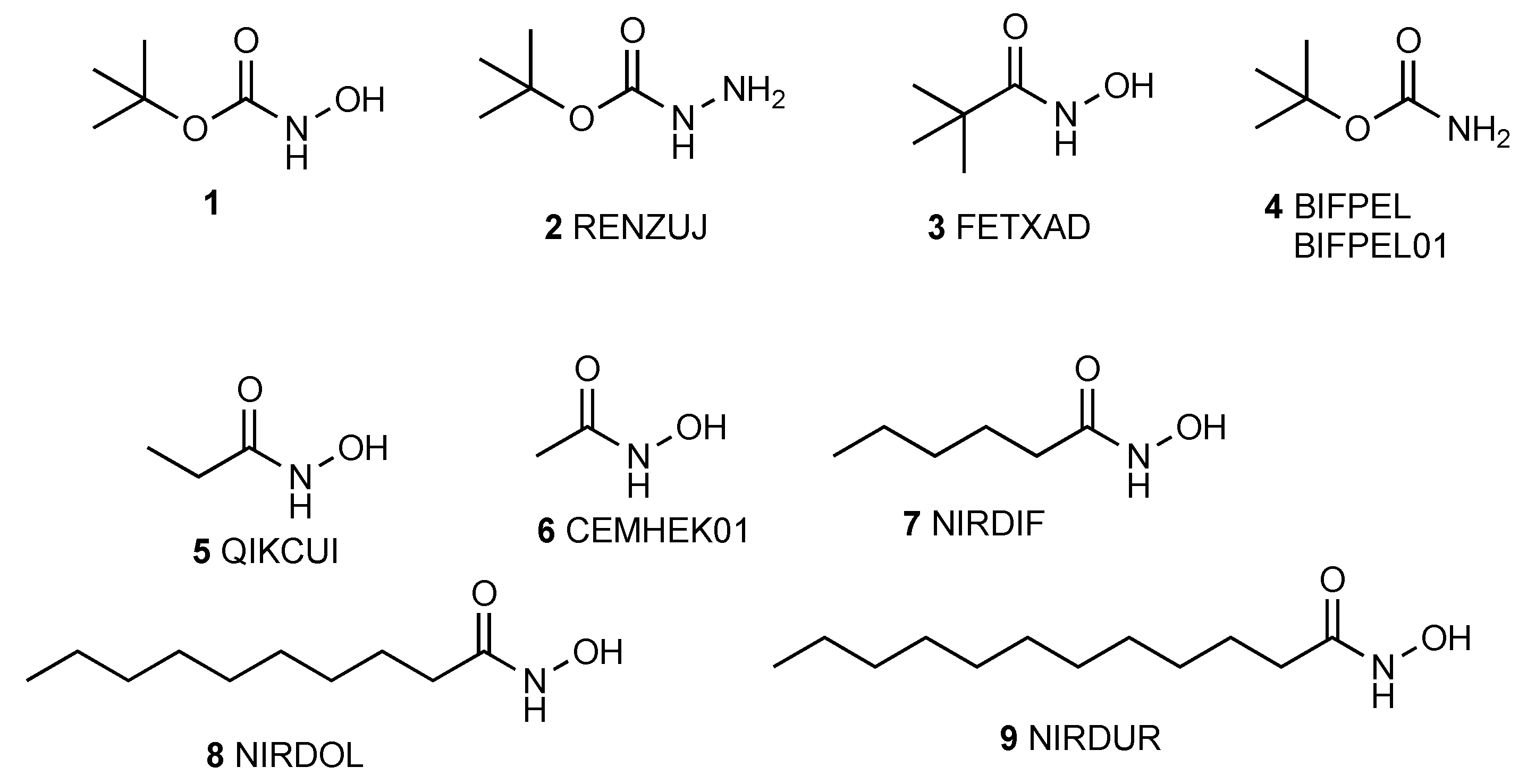
:1. Introduction
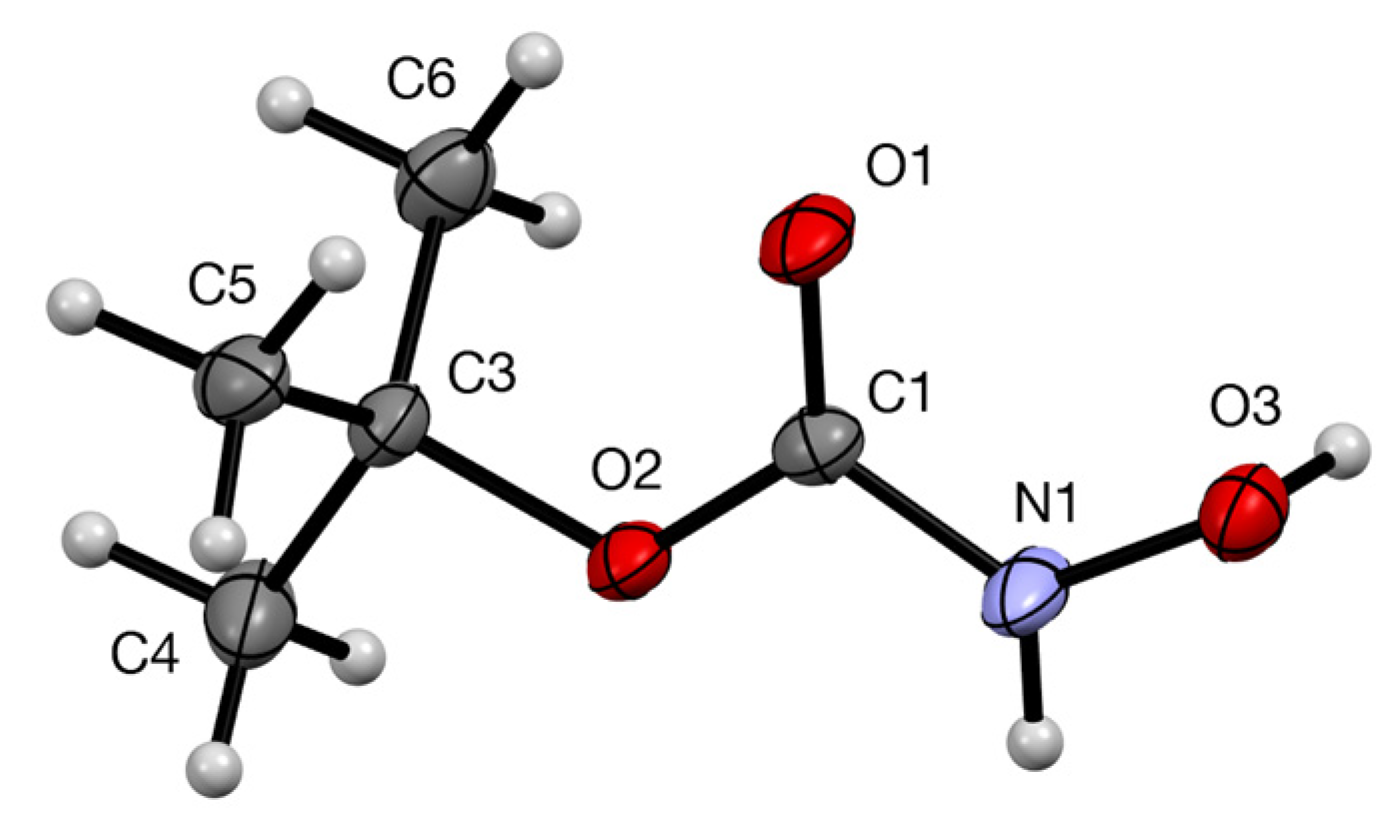
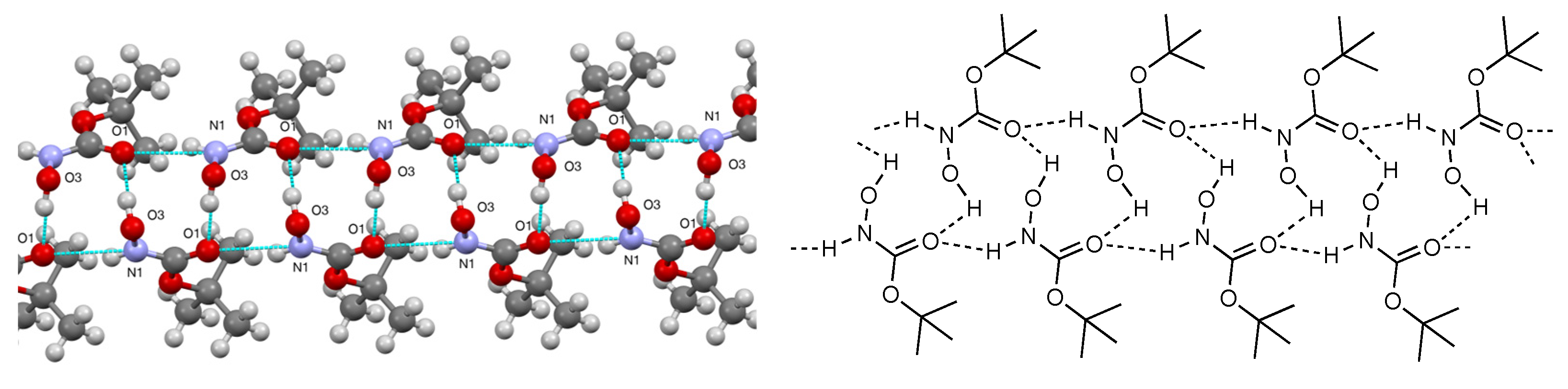
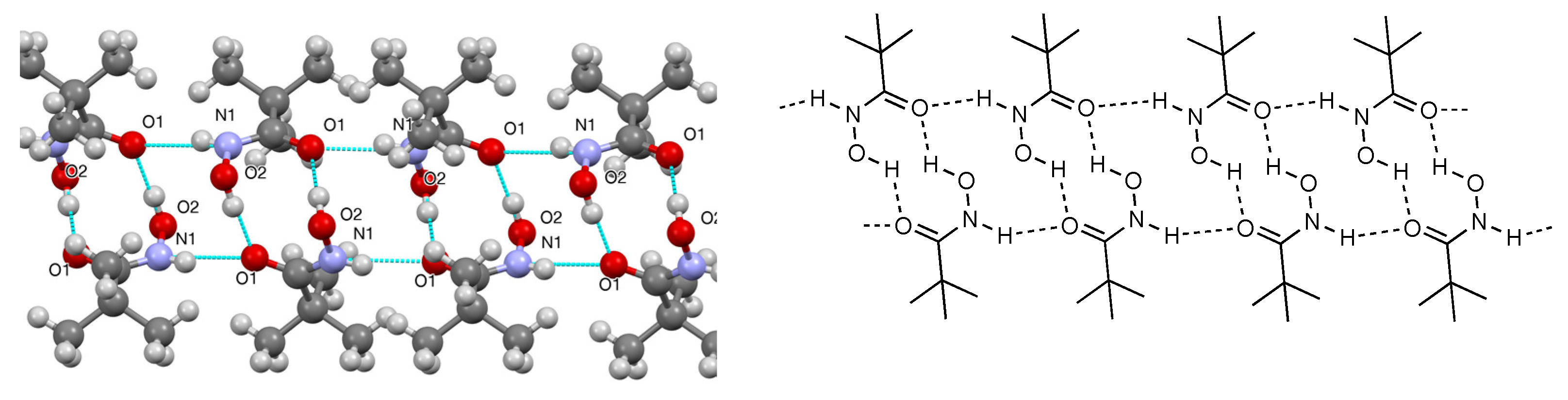
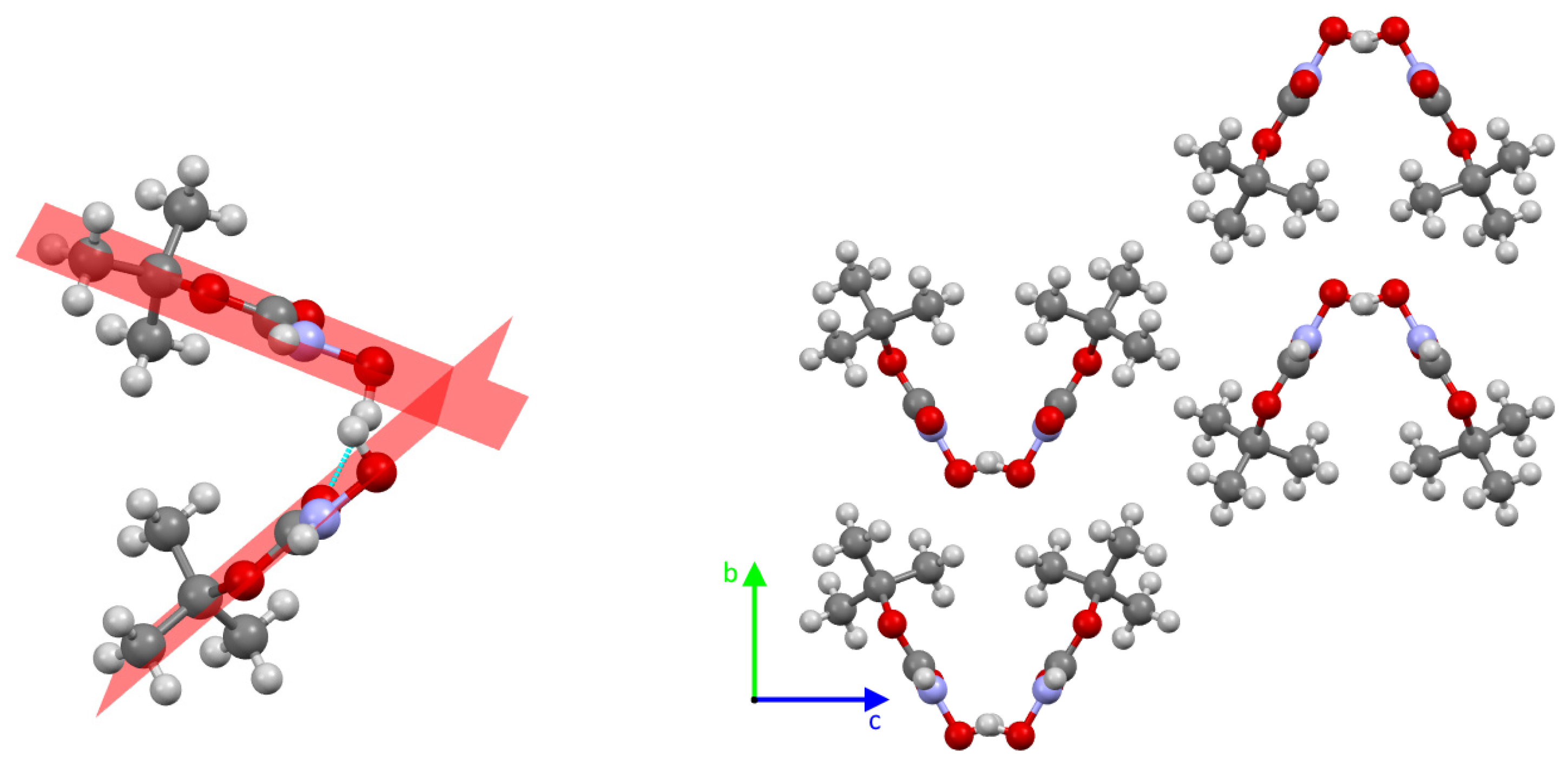
2. Results
3. Experimental Section
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carpino, L.A.; Giza, C.A.; Carpino, B.A. O-Acylhydroxylamines I. Synthesis of O-benzoylhydroxylamine. J. Am. Chem. Soc. 1959, 81, 955–957. [Google Scholar] [CrossRef]
- Fährmann, J.; Hermann, L.; Hilt, G. The application of 1,2-oxazinanes as chiral cyclic Weinreb amide-type auxiliaries leading to a three-component, one-pot reaction. Synthesis 2022, 54, 2005–2018. [Google Scholar] [CrossRef]
- Aitken, R.A.; Slawin, A.M.Z. tert-Butyl carbazate (N-Boc-hydrazine). Molbank 2022, 2022, M1482. [Google Scholar] [CrossRef]
- Due, L.; Rasmussen, H.; Larsen, I.K. Molecular structures of ribonucleotide reductase inhibitors: 3,4-dihydroxybenzohydroxamic acid monohydrate and pivalohydroxamic acid. Acta Crystallogr. Sect. C 1987, 43, 582–585. [Google Scholar] [CrossRef]
- CSD Entry: BIFPEL. Available online: https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=bifpel&DatabaseToSearch=Published (accessed on 30 August 2023).
- Bursavich, M.G.; Fronczek, F.R. tert-Butyl carbamate at 100K. Acta Crystallogr. Sect. C 1997, 53, IUC9700022. [Google Scholar] [CrossRef]
- Ferguson, G.; Glidewell, C. N-Propionylhydroxylamine forms a three-dimensional hydrogen-bonded framework structure. Acta Crystallogr. Sect. C 2001, 57, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Sow, I.S.; Gelbcke, M.; Dufrasne, F.; Robeyns, K. Crystal structures of a series of hydroxamic acids. Molbank 2023, 2023, M1367. [Google Scholar] [CrossRef]
- Pantoom, S.; Hules, L.; Schöll, C.; Petrosyan, A.; Monticelli, M.; Pospech, J.; Cubellis, M.V.; Hermann, A.; Lukas, J. Mechanistic insight into the mode of action of acid β-glucosidase enhancer ambroxol. Int. J. Mol. Sci. 2022, 23, 3536. [Google Scholar] [CrossRef] [PubMed]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-Set Analysis of Hydrogen-Bond Patterns in Organic Crystals. Acta Crystallogr. Sect. B 1990, 46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Rigaku, O.D. CrysAlisPro; v1.171.42.94a; Rigaku Oxford Diffraction; Rigaku Corporation: Tokyo, Japan, 2023. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Cordes, D.B.; McKay, A.P.; Sonecha, D.K. tert-Butyl N-Hydroxycarbamate (N-Boc-Hydroxylamine). Molbank 2023, 2023, M1728. https://doi.org/10.3390/M1728
Aitken RA, Cordes DB, McKay AP, Sonecha DK. tert-Butyl N-Hydroxycarbamate (N-Boc-Hydroxylamine). Molbank. 2023; 2023(3):M1728. https://doi.org/10.3390/M1728
Chicago/Turabian StyleAitken, R. Alan, David B. Cordes, Aidan P. McKay, and Dheirya K. Sonecha. 2023. "tert-Butyl N-Hydroxycarbamate (N-Boc-Hydroxylamine)" Molbank 2023, no. 3: M1728. https://doi.org/10.3390/M1728
APA StyleAitken, R. A., Cordes, D. B., McKay, A. P., & Sonecha, D. K. (2023). tert-Butyl N-Hydroxycarbamate (N-Boc-Hydroxylamine). Molbank, 2023(3), M1728. https://doi.org/10.3390/M1728








