Abstract
2,7-Bis(pyridin-4-ylethynyl)-9H-carbazole (1) was synthesized by reacting 4-ethynylpyridine hydrochloride with 2,7-dibromo-9H-carbazole. The full characterization of compound 1 is presented, and the crystal structure of its monohydrate was determined by single-crystal XRD analysis.
1. Introduction
Over the past decades, extensive synthetic efforts have led to the fabrication of innovative building blocks with different fluorophore cores such as fluorene [1], carbazole [2], and thiophene [3] in view of producing supramolecular scaffolds with tunable luminescence properties for applications in optoelectronics [4], sensing [5], and bioimaging [6]. Among the neutral carbazole-based building blocks reported so far, pyridyl derivatives such as the bent 3,6-di(pyridin-4-yl)-9H-carbazole [3,6-(4-Py)2Cz] (Scheme 1) have been successfully incorporated into discrete and polymeric supramolecular assemblies showing interesting properties as pH probes [7] and sensors for both cations and anions [8] by exploiting the intrinsic electron-rich properties of the carbazolyl core that resulted in spectroscopically active networks.
By extending the size of 3,6-(4-Py)2Cz through the inclusion of ethynyl functionalities, 3,6-bis(pyridin-4-ylethynyl)-9H-carbazole [3,6-(4-PyE)2Cz] (Scheme 1) was prepared, showing its value in the formation of rare architectures such as a Solomon link that was recently reported, in which the authors emphasized the role of ethynyl moieties in establishing additional π–π interactions [9].
Passing from the bent 3,6-carbazolyl to the almost linear 2,7-carbazolyl core, the synthesis of 2,7-bis(pyridin-4-yl)-9H-carbazole [2,7-(4-Py)2Cz] was also reported, and this compound was used as a donor for the preparation of supramolecular wires and rectangles along with its 9H-fluorene and fluoren-9-one congeners, namely, 2,7-di(pyridin-4-yl)-9H-fluorene [2,7-(4-Py)2Fl] and 2,7-bis(pyridin-4-yl)fluoren-9-one [2,7-(4-Py)2FO] (Scheme 1) [10].
Herein, we report on the synthesis of 2,7-bis(pyridin-4-ylethynyl)-9H-carbazole (1) by following our previous reports on pyridyl-ethynyl derivatives, namely, 2,7-bis(pyridin-3-ylethynyl)thiophene [11] [2,7-(3-PyE)2Tp], 2,7-bis(pyridin-3-ylethynyl)fluoren-9-one [2,7-(3-PyE)2FO] [12], and 2-(2,7-bis(pyridin-3-ylethynyl)fluoren-9-ylidene)malononitrile [2,7-(3-PyE)2Fmnt] [13] (Scheme 1).
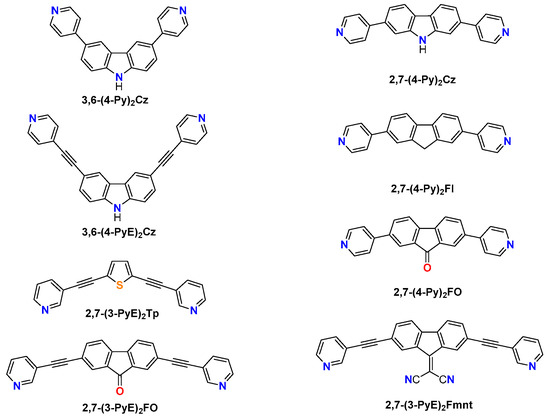
Scheme 1.
Selected pyridyl derivatives: 3,6-(4-Py)2Cz [7,8], 3,6-(4-PyE)2Cz [9], 2,7-(4-Py)2Cz [10], 2,7-(4-Py)2Fl [10], 2,7-(4-Py)2FO [10], 2,7-(3-PyE)2Tp [11], 2,7-(3-PyE)2FO [12], and 2,7-(3-PyE)2Fmnt [13].
2. Results and Discussion
Compound 1 was synthesized by reacting a slight excess of 4-ethynylpyridine hydrochloride (2.2 eq.) with 2,7-dibromo-9H-carbazole in a mixture of dry triethylamine/toluene under an inert atmosphere via the Sonogashira coupling (Scheme 2). After column-chromatographic purification, the product was thoroughly characterized by elemental analysis, FT-IR, high-resolution ESI-MS, and NMR techniques (Figures S1–S3). The alkyne stretching vibration was observed at 2208 cm−1, similar to other pyridyl-ethynyl derivatives [11,12,13]. The high-resolution mass spectrum of compound 1 showed the monoprotonated peak at m/z 370.1328 as well as the double-charged peak at m/z 185.5699 (Figure S4). The 1H NMR spectrum of 1 in DMSO-d6 showed signals in the range of 11.69–7.42 ppm, with the NH proton resonating at 11.69 ppm and the carbazolyl protons centered at 8.25, 7.79, and 7.43 ppm, while the corresponding protons of the para-substituted pyridyl moieties were found at 8.66 and 7.57 ppm. The 13C NMR spectrum recorded in the same solvent is consistent with the chemical structure of 1, with carbon signals distributed over the range 150–87 ppm and the two most upfield signals resonating at 87.1 and 95.5 ppm, respectively, ascribable to the quaternary carbons of the ethynyl groups.
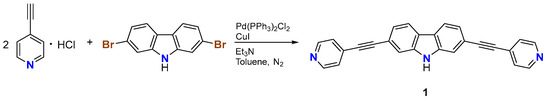
Scheme 2.
Synthesis of 2,7-bis(pyridin-4-ylethynyl)-9H-carbazole (1).
Crystal data for 1∙H2O: C26H17N3O (Mr = 387.42 g mol−1) monoclinic, P21/n (No. 14), a = 9.0349(3) Å, b = 25.4077(7) Å, c = 17.7142(4) Å, β = 97.260(3)°, α = γ = 90°, V = 4033.8(2) Å3, T = 100(2) K, Z = 8, Z’ = 2, µ(Cu Kα) = 0.629 mm−1, 37,690 reflections measured, 7385 unique (Rint = 0.0562), which were used in all calculations. The final wR2 was 0.2459 (all data) and R1 was 0.0907 (I > 2(I)).
Compound 1 was recrystallized from a 3:1 DMSO/H2O mixture as colorless crystals whose structure was unambiguously elucidated by single-crystal X-ray diffraction analysis as the monohydrate form of 1. The compound 1∙H2O crystallized in the monoclinic P21/n space group with two crystallographically independent molecules of 1 in the asymmetric unit coupled with two water molecules that are hydrogen-bonded to pyridyl groups (Figure 1).
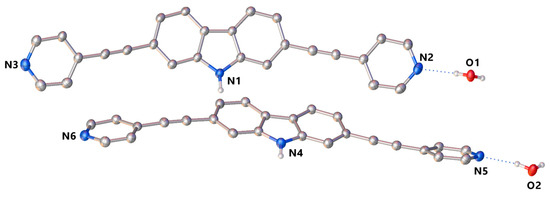
Figure 1.
X-ray crystal structure of 1∙H2O with thermal ellipsoids drawn at a 30% probability level. Only H-atoms located on heteroatoms and water molecules are shown for clarity.
The two symmetry-independent carbazole molecules are nearly planar, with the terminal pyridyl rings rotated by angles ranging between 3° and 25°. It is worth noting that the rotational barrier for pyridyl groups in similar derivatives was calculated to be a few kcal·mol−1 [11,14], suggesting that the overall conformation adopted by the donor in the solid-state is likely to be easily modulated and governed by supramolecular interactions.
The oxygen atoms of water molecules behave as three-connecting nodes between donor units of 1, acting either as hydrogen bond donors via OH∙∙∙N hydrogen bonds with pyridyl rings (shortest dD∙∙∙A = 2.786(4) Å) or as hydrogen bond acceptors through NH∙∙∙O interactions with carbazole protons (shortest dD∙∙∙A = 2.789(4) Å). The repetition of the aforementioned interactions within the crystal lattice results in the packing of pairs of waved H-bonded chains running along the b-axis, as shown in Figure 2.
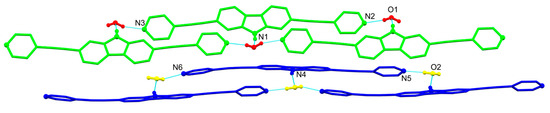
Figure 2.
View along the c-axis of the hydrogen-bonded chains found in 1∙H2O with moieties colored according to their symmetry equivalence.
3. Materials and Methods
3.1. General
Solvents and reagents were purchased from VWR, TCI, FluoroChem, and Merck. Triethylamine was distilled over LiAlH4 and degassed by three consecutive freeze-pump-thaw cycles prior to use. Toluene was distilled over Na and stored with molecular sieves. The synthesis of 1 was carried out under a dry dinitrogen atmosphere using standard Schlenk equipment.
FT-IR measurements were recorded at room temperature on a Thermo-Nicolet 5700 spectrometer using KBr pellets with a KBr beam splitter and KBr windows (4000−400 cm−1, resolution 4 cm−1). NMR spectra were carried out in DMSO-d6 at room temperature on a Bruker Avance III HD 600 spectrometer. Chemical shifts are reported in ppm (δ) and were calibrated to the solvent residue. Coupling constants J are given in Hz units. Positive ESI-MS spectra were recorded on a high-resolution LTQ Orbitrap Elite™ mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The solutions were infused into the ESI source at a flow rate of 5.00 μL/min. Spectra were recorded in the range of m/z 300–600 with a resolution of 240,000 (FWHM). Instrument conditions were as follows: spray voltage 3500 V, capillary temperature 275 °C, sheath gas 12 (arbitrary units), auxiliary gas 3 (arbitrary units), sweep gas 0 (arbitrary units), probe heater temperature 50 °C. Elemental analysis was performed with a CHNS/O PE 2400 series II elemental analyzer (T = 925 °C). The melting point was determined on a FALC mod. C apparatus (up to 290 °C).
X-ray diffraction data for 1·H2O were collected at 100(2) K on a Rigaku 007HF diffractometer, equipped with Varimax confocal mirrors, an AFC11 goniometer, and a HyPix 6000 detector. The structure was solved with the ShelXT [15] solution program using dual methods, and the model was refined with ShelXL 2018/3 [16] using full matrix least squares minimization on F2. Olex2 1.5 [17] was used as the graphical interface. All crystals screened were twinned; therefore, data were collected on the crystal that showed the least observable amount of twinning. It was not possible to integrate and account for the X-ray diffraction of the minor twin component, thus lowering the quality statistics of the refinement. The water molecules were modeled and refined as ridged bodies with idealized geometries, as they refined to unrealistic geometries.
3.2. Synthesis of 2,7-Bis(pyridin-4-ylethynyl)-9H-carbazole (1)
2,7-Dibromo-9H-carbazole (0.325 g; 1.00 mmol), 4-ethynylpyridine hydrochloride (0.307 g; 2.20 mmol), copper(I) iodide (0.019 g; 0.10 mmol), and Pd(PPh3)2Cl2 (0.070 g; 0.10 mmol) were added to a 50 mL three-necked round bottom flask under dinitrogen atmosphere. Freshly distilled and degassed triethylamine (5 mL) and dry toluene (15 mL) were added via cannula, and the mixture was heated to reflux for 48 h. After cooling to 0 °C, the black precipitate was filtered under reduced pressure and purified by flash column chromatography on silica gel using a 2:1 CH2Cl2/ethyl acetate mixture as eluent. The pure product was isolated as an off-white solid (0.072 g; Y = 19%). M.p. = 230 °C. Elemental analysis calcd (%) for C26H15N3: C 84.53, H 4.09, N 11.37. Found: C 84.34, H 3.61, N 11.05. HR-ESI(+)-MS (MeCN solution) m/z: 370.1328 (calcd. 370.1333) for [C26H16N3]+ [M + H]+; m/z: 185.5699 (calcd. 185.5706) for [C26H17N3]2+ [M + 2H]2+. FT-IR (KBr, 4000–400 cm−1): 3427s ν(N−H), 2208m ν(C≡C), 1628m, 1593m, 1537w, 1479w, 1439mw, 1408mw, 1385ms, 1329m, 1244w, 1209w, 997w, 872mw, 812ms, 808ms, 731w, 633w, 544w, 467w, 418w cm−1. 1H NMR (600 MHz, DMSO-d6) δ 11.69 (s, 1H, NH), 8.66 (s, 4H, py), 8.26 (d, J = 8.1 Hz, 2H, Cz), 7.79 (s, 2H, Cz), 7.58 (d, J = 5.0 Hz, 4H, py), 7.43 (d, J = 8.1 Hz, 2H, Cz) ppm. 13C{1H} NMR (151 MHz, DMSO-d6) δ: 150.4, 140.6, 130.9, 125.9, 123.3, 123.2, 121.7, 119.0, 115.1, 95.5, 87.1 ppm.
4. Conclusions
The donor 2,7-bis(pyridin-4-ylethynyl)-9H-carbazole (1) was successfully prepared, and its monohydrate form was structurally characterized by crystallographic means. Further studies are ongoing in our laboratories to explore the supramolecular chemistry of 1 towards various complementary building blocks.
Supplementary Materials
The following supporting information can be downloaded, Figure S1: FT-IR spectrum; Figures S2 and S3: 1H and 13C{1H} NMR spectra; Figure S4: HR-ESI(+) MS spectrum; Table S1: Crystal data and refinement parameters; Tables S2 and S3: Bond lengths and angles.
Author Contributions
Conceptualization: M.C.A. and E.P. Data curation: M.C.A., E.P., A.P., M.A., J.B.O. and S.J.C. Investigation: M.C.A., E.P., M.A., V.L., G.F., S.J.C., J.B.O. and A.P. Writing (original draft): M.C.A. and E.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge Fondazione di Sardegna (FdS Progetti Biennali di Ateneo, annualità 2018) for financial support and to the EPSRC (Engineering and Physical Science Research Council) for continued support of the UK’s National Crystallography Service (NCS), based at the University of Southampton.
Data Availability Statement
Crystallographic data were deposited at CCCD (CIF deposition number 2263922).
Acknowledgments
CeSAR (Centro Servizi di Ateneo per la Ricerca) of the University of Cagliari is kindly acknowledged for NMR and MS facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Podda, E.; Arca, M.; Pintus, A.; Lippolis, V.; Caltagirone, C.; Coles, S.; Orton, J.B.; Ennas, G.; Picci, G.; Davies, R.; et al. On the Role of Torsional Dynamics in the Solid-State Fluorescent Properties of a New Bifluorene-Tetracarboxylic Acid and Its Supramolecular Assemblies: A Structural and TD-DFT Investigation. CrystEngComm 2023, 25, 1058–1066. [Google Scholar] [CrossRef]
- Ma, T.; Li, K.; Hu, J.; Xin, Y.; Cao, J.; He, J.; Xu, Z. Carbazole-Equipped Metal-Organic Framework for Stability, Photocatalysis, and Fluorescence Detection. Inorg. Chem. 2022, 61, 14352–14360. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.C.; Evenson, S.J.; McCausland, C.B. Fluorescent Thiophene-Based Materials and Their Outlook for Emissive Applications. Chem. Commun. 2015, 51, 4528–4543. [Google Scholar] [CrossRef] [PubMed]
- Konidena, R.K.; Thomas, K.R.J.; Park, J.W. Recent Advances in the Design of Multi-Substituted Carbazoles for Optoelectronics: Synthesis and Structure-Property Outlook. ChemPhotoChem 2022, 6, e202200059. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, X.; Zhang, L.; Eubank, J.F.; Liu, X.; Liu, Y. Unique Fluorescence Turn-On and Turn-Off-On Responses to Acids by a Carbazole-Based Metal-Organic Framework and Theoretical Studies. J. Am. Chem. Soc. 2022, 144, 17054–17063. [Google Scholar] [CrossRef]
- Palamà, I.; Di Maria, F.; Viola, I.; Fabiano, E.; Gigli, G.; Bettini, C.; Barbarella, G. Live-Cell-Permeant Thiophene Fluorophores and Cell-Mediated Formation of Fluorescent Fibrils. J. Am. Chem. Soc. 2011, 133, 17777–17785. [Google Scholar] [CrossRef]
- Chuang, P.M.; Wu, J.Y. A Highly Stable Zn Coordination Polymer Exhibiting pH-Dependent Fluorescence and as a Visually Ratiometric and on–off Fluorescent Sensor. CrystEngComm 2021, 23, 5226–5240. [Google Scholar] [CrossRef]
- Chuang, P.M.; Huang, Y.W.; Liu, Y.L.; Wu, J.Y. The Influence of Linker Substitution on the Fluorescence Responsive Sensing of Isostructural Coordination Polymers: Visual Turn-on, Ratiometric, and Turn-off Sensing in Water. CrystEngComm 2021, 23, 2222–2234. [Google Scholar] [CrossRef]
- Song, Y.H.; Singh, N.; Jung, J.; Kim, H.; Kim, E.H.; Cheong, H.K.; Kim, Y.; Chi, K.W. Template-Free Synthesis of a Molecular Solomon Link by Two-Component Self-Assembly. Angew. Chem. Int. Ed. 2016, 55, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, Z.K.; Lang, F.F.; Yu, H.M.; Cao, C.; Ni, C.Y.; Wang, M.Y.; Song, Y.L.; Lang, J.P. Construction of Cluster-Based Supramolecular Wire and Rectangle. Dalton Trans. 2022, 51, 6358–6365. [Google Scholar] [CrossRef] [PubMed]
- Podda, E.; Coles, S.J.; Horton, P.N.; Lickiss, P.D.; Bull, O.S.; Orton, J.B.; Pintus, A.; Pugh, D.; Aragoni, M.C.; Davies, R.P. First Example of Solid-State Luminescent Borasiloxane-Based Chiral Helices Assembled through N-B Bonds. Dalton Trans. 2021, 50, 3782–3785. [Google Scholar] [CrossRef]
- Podda, E.; Arca, M.; Pintus, A.; Demontis, V.; Lippolis, V.; Ferino, G.; Orton, J.B.; Coles, S.J.; Aragoni, M.C. 2,7-Bis(Pyridin-3-Ylethynyl)Fluoren-9-One. Molbank 2023, 2023, M1540. [Google Scholar] [CrossRef]
- Podda, E.; Arca, M.; Pintus, A.; Meloni, F.; Lippolis, V.; Ferino, G.; Orton, J.B.; Coles, S.J.; Aragoni, M.C. 2-(2,7-Bis(Pyridin-3-Ylethynyl)Fluoren-9-Ylidene)Malononitrile. Molbank 2023, 2023, M1619. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Coles, S.J.; Crespo Alonso, M.; Coles, S.L.; Davies, R.P.; Hursthouse, M.B.; Isaia, F.; Lai, R.; Lippolis, V. Coordination Polymers and Polygons Using Di-Pyridyl-Thiadiazole Spacers and Substituted Phosphorodithioato NiII Complexes: Potential and Limitations for Inorganic Crystal Engineering. CrystEngComm 2016, 18, 5620–5629. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).