Abstract
This article presents a novel approach for synthesizing a new 5,8-dimethoxy derivative of esculetin via the selective cleavage of the methylene bridge in sabandin—naturally occurring and easily synthetically accessible methoxylated coumarin. A high selectivity is achieved by using acetoxylation of methylenedioxy group with lead tetraacetate. Natural coumarin sabandin as a starting compound was prepared in a few simple steps from 5-allyl-4,7-dimethoxybenzo[d][1,3]dioxole (apiol), which is readily available from parsley and dill seed extracts. The developed method enables an efficient and straightforward synthesis of a new derivative of esculetin with potential medicinal and therapeutic applications.
1. Introduction
Coumarins are a class of naturally occurring compounds that have been found to possess a wide range of biological activities [1,2]. In particular, oxygenated derivatives of coumarins have been identified as biologically active substances in many medicinal plants [3]. These compounds have been shown to exhibit antioxidant, anti-inflammatory, anti-apoptotic, anticancer, antidiabetic, neuroprotective, and cardiovascular protective activities, in addition to antibacterial activity [4]. Pelargonium sidoides is a prime illustration of a medicinal herb that contains coumarins. The polyalkoxysubstituted coumarins found in P. s. roots have been utilized to address respiratory and gastrointestinal infections [5]. Another natural coumarin, 6,7-dihydroxy-2H-chromen-2-one (esculetin) 1, has shown promise as a therapeutic drug for specific diseases such as cancer, diabetes, atherosclerosis, Alzheimer’s disease, Parkinson’s disease, and others [6]. In addition to biological activity, 6,7-dihydroxycoumarins also possess a high synthetic potential to obtain of diverse and complicated biologically active compounds [7].
2. Results and Discussion
In order to synthesize new multifunctional coenzyme Q0 analogs, we needed an accessible approach to 6,7-dihydroxy-5,8-dimethoxy-2H-chromen-2-one 2. This compound can be considered as a derivative of both natural coumarins esculetin 1 and sabandin 3. The chemical structure of 2 differs from sabandin 3 due to the absence of a methylene bridge connecting two 6,7-phenolic groups and also differs from esculetin 1 by two additional methoxy groups at positions 5 and 8 (Scheme 1). Interestingly, despite its resemblance to naturally occurring 1 and 3, coumarin 2 has not yet been identified in any plant source [8].
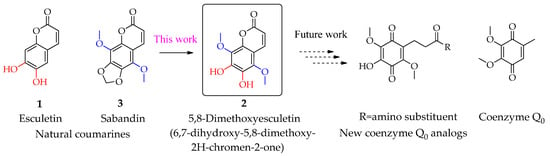
Scheme 1.
5,8-Dimethoxyesculetin (6,7-dihydroxy-5,8-dimethoxy-2H-chromen-2-one) 2-precursor for synthesis of new coenzyme Q0 analogs.
To synthesize our target compound 2, we utilized a readily available starting material with highly oxygenated benzene core—4,7-dimethoxybenzo[d][1,3]dioxole-5-carbaldehyde 4 [9]. This compound was obtained from apiol 5, which is easily isolated in large quantities from CO2 extracts of parsley and dill seeds [10]. The synthesis of 3 involved several modified literature steps, including a SeO2-catalyzed Baeyer–Villiger reaction to obtain phenol 6 [11], followed by its formylation to aldehyde 7 [9] and finally Wittig condensation accompanied with lactonization to obtain a natural coumarin sabandin 3 [12] with an overall yield of 70% (Scheme 2).
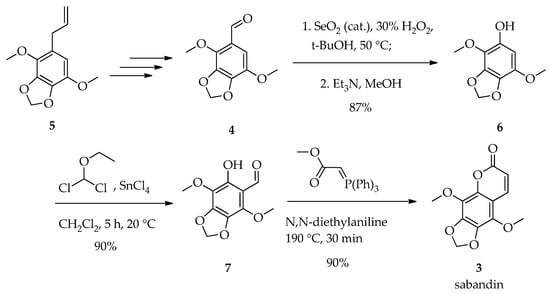
Scheme 2.
Synthesis of sabandin 3 starting from 4,7-dimethoxybenzo[d][1,3]dioxole-5-carbaldehyde 4.
To our best knowledge, there is only one example of the methylene bridge cleavage in coumarins with a fused dioxole ring [13]. This procedure involves the usage of aluminum tribromide in ethanethiol—hazardous and toxic reagents. Unfortunately, it was not suitable for our purposes, since it does not allow the cleavage of O-C-O bonds selectively without affecting the methoxy groups. Searching for alternative methods, we found that the methylene bridge can be selectively oxidized using Pb(OAc)4. Despite that lead tetraacetate is a common oxidizing agent, its use for methylenedioxy group cleavage is described for very few cases [14,15]. The transformation involves selective free radical oxidation of the methylene bridge, forming the corresponding acetoxy derivative, which readily undergoes an acid hydrolysis to form the final dihydroxy product [16].
By treatment of coumarin 3 with Pb(OAc)4 followed by hydrolysis of resulting acetate 8 with an HCl-i-PrOH solution, we were able to obtain the dihydroxycoumarin 2 while retaining the existing methoxy groups, with minimal side processes and a total isolated yield of 80% in two stages (Scheme 3). The oxidation 3 to 8 is slow (60 h) but selective, and its course can be monitored by detecting lead tetraacetate during the reaction. It was observed that control of the reaction using thin-layer chromatography (TLC) was arduous due to the same Rf values of the substrate 3 and acetate intermediate 8 (~0.36 in cyclohexane-ethyl acetate 2:1). However, it was discovered that the 3 appears blue, and the intermediate 8 exhibits a red color upon treatment of the TLC plate with an acidic cerium sulfate solution. In addition, the presence of Pb(OAc)4 in the mixture can be determined by a simple chemical test: a drop of the reaction mixture solution is applied to wet filter paper. If there is a lot of tetraacetate in the mixture, the stain immediately turns black from the lead dioxide formed during hydrolysis. As the reagent is consumed, the spot becomes less apparent, and when no darkening is observed, the reaction is close to completion. The final control is made by NMR analysis of the selected sample, which indicated complete conversion of the starting compound 3. Due to the low stability of the resulting acetate 8, it was deemed preferable to carry it forward to the next step without isolation. While the hydrolysis of 8 under acetic acid proved to be a slow process, a solution of hydrogen chloride in isopropanol was observed to react more promptly and efficiently. A part of the product of 2 precipitates from the reaction mixture, and the remainder is isolated by crystallization from isopropanol.
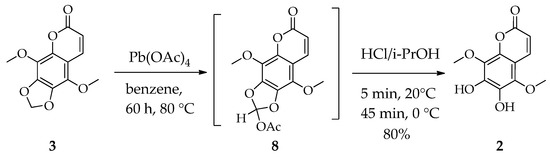
Scheme 3.
Oxidation of sabandin 3 and hydrolysis of intermediate acetoxy derivative 8 to 5,8-dimethoxyesculetine 2.
The 1H and 13C NMR spectra confirm the structure of compound 2 (Figures S1 and S2). Signals of two methoxy groups and coumarin double bonds are present. The absence of methylenedioxy moiety signals clearly indicates its successful removal upon the action of Pb(OAc)4. The IR spectrum (Figure S4) shows a broad band at 3397 cm−1 that is characteristic for phenolic groups. Mass spectrum (Figure S3) of 2 confirms the molecular weight 238 and the presence of the fragment (MW − CH3 = 223).
3. Materials and Methods
3.1. General Information
The melting points were determined on a Nagema PHMK 05 polarizing microscope with a Boetius heating stage. 1H and 13C NMR spectra were recorded in DMSO-d6 on Bruker DRX500 (operating frequency 500.13 MHz for 1H and 125.76 MHz for 13C) and Bruker AV600 SF spectrometers (operating frequency 600.13 MHz for 1H). Chemical shifts in the 1H NMR spectra are given relative to the residual proton signal of the solvent (DMSO − δH 2.50 ppm), in the 13C NMR spectra—relative to the solvent signal (DMSO − δC 39.5 ppm). Mass spectra (m/z) were recorded on a Finnigan MAT/INCOS 50 mass spectrometer at 70 eV using direct input. High resolution mass spectra (HRMS) were obtained on a Bruker micrOTOF 10,248 instrument by electrospray ionization (ESI). The IR spectrum was recorded with a Bruker (Moscow, Russia) “Alpha-T” instrument. Reactions and product mixtures were routinely monitored by TLC on silica gel 60 F254 Merck plates, and compounds were visualized under UV light or with aqueous Ce(SO4)2-H2SO4 and heat as developing agents. Products were purified by recrystallization or silica column chromatography using 0.060–0.200 mm silica gel (Acros) and the indicated solvent system. All solvents are distilled prior to use. Organic solutions were dried over anhydrous MgSO4.
3.2. Synthesis of 6,7-Dihydroxy-5,8-dimethoxy-2H-chromen-2-one (2)
A mixture of 3 (18.52 g, 74 mmol) and Pb(OAc)4 (95%, 51.80 g, 111 mmol) in benzene (350 mL) was placed in a three-necked 500 mL flask equipped with a calcium chloride tube and a thermometer, and the resulting brown solution was stirred at 80 °C for 60 h. After 6 h, Pb(OAc)2 began to precipitate from the mixture. As the reaction proceeded, the color of the mixture changed from brown to yellow. Every 10–15 h, a drop of the reaction mixture was placed on wet filter paper, and the color was checked. At the beginning of the reaction, the spot turned black, and by the end, when almost all Pb(OAc)4 had been consumed, no blackening occurred. After 50 h, 1 mL of the reaction solution was taken, diluted with saturated NaHCO3, extracted with CH2Cl2, and evaporated, and the 1H NMR spectrum was recorded. The content of the starting material, measured by the signal of the methylenedioxy group (2H, 6.13 ppm), was about 2%. Then, the mixture was stirred at 80 °C for another 10 h to complete the reaction. After that, the reaction mixture was cooled, and the precipitate of Pb(OAc)2 was filtered off and washed with benzene. The filtrate was evaporated to about 100 mL, and 300 mL of water and of NaHCO3 (9.32 g, 111 mmol) was added to the black residue (pH = 6.5). The organic layer was separated, and the aqueous layer with a black precipitate was washed with 3 × 50 mL of benzene. The organic layer was dried and evaporated in vacuum. The aqueous phase was additionally extracted with CH2Cl2 (1 × 50 mL); extracts were combined with the main fraction, dried, and evaporated. The orange amorphous residue was dried to a constant weight at 0.1 mbar, to give 22.54 g (99%) of acetate 8 with a small amount of impurities. It was dissolved in 100 mL of CH2Cl2 and 10 mL of i-PrOH, forming a red solution. Then, 40 mL HCl-i-PrOH (9 M, 360 mmol) was added at room temperature. The solution turned dark. After a few minutes, 2 began to precipitate from the mixture. The mixture was cooled in an ice-water bath, and after 40 min the precipitate was filtered off and washed with i-PrOH, and the product was purified by crystallization from i-PrOH, giving 6.79 g of 2. An additional 7.32 g of 2 was isolated from the evaporated mother liquors by repeated recrystallization. A total of 14.11 g of 2 was obtained, with a yield of 80%.
6,7-Dihydroxy-5,8-dimethoxy-2H-chromen-2-one (2): light beige crystals (i-PrOH); mp 222−223 °C; IR (KBr) νmax 3397, 2949, 2842, 1707, 1615, 1578, 1490, 1415, 1286, 1160, 1041, 1005, 933, 823, 798; 1H NMR δ 10.04 (1H, br s, OH), 9.21 (1H, br s, OH), 7.97 (1H, d, J = 9.6 Hz, H-4), 6.21 (1H, d, J = 9.6 Hz, H-3), 3.81 (3H, s, OCH3), 3.80 (3H, s, OCH3); 13C NMR δ 160.1 (C, C-2), 144.7 (C, C-7), 140.9 (C, C-8a), 139.6 (CH, C-4), 139.3 (C, C-5), 135.6 (C, C-6), 131.2 (C, C-8), 111.3 (CH, C-3), 105.2 (C, C-4a), 61.4 (CH3), 60.9 (CH3); EIMS m/z 238 [M]+ (100), 223 (79), 177 (2), 167 (8), 152 (3), 139 (7), 111 (2), 95 (3), 83 (7); HRMS (ESI-TOF) m/z 239.0550 (calcd for C11H11O6 [M + H]+, 239.0550).
4. Conclusions
A straightforward procedure for the synthesis of unnatural 5,8-dimethoxyesculetin 2 starting from 4,7-dimethoxybenzo[d][1,3]dioxole-5-carbaldehyde 4 was developed with a total yield of 56% after 7 stages. We have found that the use of lead tetraacetate makes it possible to selectively solve the problem of methylene bridge cleavage in the presence of methoxy groups in available coumarin compounds.
Supplementary Materials
The following supporting information is available online, Experimental procedures: Compounds 3,6,7; Figures S1–S5: 1H NMR, 13C NMR, Mass, IR and HRMS spectra for compound 2; Figures S6–S10: 1H NMR, 13C NMR, Mass, IR and HRMS spectra for compound 3; Figures S11–S13: 1H NMR, 13C NMR and Mass spectra for compound 6; Figures S14–S16: 1H NMR, 13C NMR and Mass spectra for compound 7.
Author Contributions
Conceptualization, O.I.A. and D.V.D.; methodology, D.V.D.; validation, O.I.A. and D.V.D.; formal analysis, O.I.A.; investigation, O.I.A. and D.V.D.; resources, D.V.D. and V.V.S.; data curation, D.V.D.; writing—original draft preparation, O.I.A.; writing—review and editing, D.V.D.; visualization, O.I.A.; supervision, V.V.S.; project administration, D.V.D. and V.V.S.; funding acquisition, V.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coumarin and Its Derivatives; Matos, M.J., Ed.; MDPI: Basel, Switzerland, 2021. [Google Scholar] [CrossRef]
- Jigar, L.P. The Introduction of Coumarin: Concise Reference Book, 1st ed.; Blue Rose Publishers: Noida, India, 2019; p. 432. [Google Scholar]
- Kayser, O. Highly oxygenated coumarins from Pelargonium sidoides. Phytochemistry 1995, 39, 1181–1185. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Cruz-Martins, N.; Lopez-Jornet, P.; Lopez, E.P.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef] [PubMed]
- Careddu, D.; Pettenazzo, A. Pelargonium sidoides extract EPs 7630: A review of its clinical efficacy and safety for treating acute respiratory tract infections in children. Int. J. Gen. Med. 2018, 11, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, Q.; Li, X. Esculetin: A review of its pharmacology and pharmacokinetics. Phytother. Res. 2022, 36, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Juang, S.H.; Hsieh, M.T.; Hsu, P.L.; Chen, J.L.; Liu, H.K.; Liang, F.P.; Kuo, S.C.; Chiu, C.Y.; Liu, S.H.; Chou, C.H.; et al. Studies of Coumarin Derivatives for Constitutive Androstane Receptor (CAR) Activation. Molecules 2020, 26, 164. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.D. The naturally occurring coumarins. In Fortschritte Der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products, 1st ed.; Herz, W., Falk, H., Kirby, G.W., Moore, R.E., Eds.; Springer: Vienna, Austria, 2002; Volume 83, pp. 1–619. [Google Scholar]
- Tsyganov, D.V.; Chernysheva, N.B.; Salamandra, L.K.; Konyushkin, L.D.; Atamanenko, O.P.; Semenova, M.N.; Semenov, V.V. Synthesis of polyalkoxy-3-(4-methoxyphenyl)coumarins with antimitotic activity from plant allylpolyalkoxybenzenes. Mendeleev Commun. 2013, 23, 147–149. [Google Scholar] [CrossRef]
- Semenov, V.V.; Rusak, V.V.; Chartov, E.M.; Zaretskii, M.I.; Konyushkin, L.D.; Firgang, S.I.; Chizhov, A.O.; Elkin, V.V.; Latin, N.N.; Bonashek, V.M.; et al. Polyalkoxybenzenes from plant raw materials 1. Isolation of polyalkoxybenzenes from CO2 extracts of Umbelliferae plant seeds. Russ. Chem. Bull. 2008, 56, 2448–2455. [Google Scholar] [CrossRef]
- Asakawa, T.; Sagara, H.; Kanakogi, M.; Hiza, A.; Tsukaguchi, Y.; Ogawa, T.; Nakayama, M.; Ouchi, H.; Inai, M.; Kan, T. Practical Synthesis of Polymethylated Flavones: Nobiletin and Its Desmethyl Derivatives. Org. Process Res. Dev. 2019, 23, 595–602. [Google Scholar] [CrossRef]
- Maes, D.; Vervisch, S.; Debenedetti, S.; Davio, C.; Mangelinckx, S.; Giubellina, N.; De Kimpe, N. Synthesis and structural revision of naturally occurring ayapin derivatives. Tetrahedron 2005, 61, 2505–2511. [Google Scholar] [CrossRef]
- Niimura, K.; Katohno, M.; Sagawa, K. Process for Preparation of Esculetin Compounds, Esculetin Compounds and Intermediates Thereof, and Use of Both. U.S. Patent US 2004/0049056A1, 11 March 2004. [Google Scholar]
- Nicolaou, K.C.; Tang, Y.; Wang, J. Total synthesis of sporolide B. Angew. Chem. Int. Ed. 2009, 48, 3449–3453. [Google Scholar] [CrossRef]
- Gross, P.J.; Brase, S. The total synthesis of (+/−)-fumimycin. Chem. Eur. J. 2010, 16, 12660–12667. [Google Scholar] [CrossRef]
- Cole, E.R.; Crank, G.; Minh, H.T.H. Oxidations with lead tetraacetate. III. Oxidations of 2-substituted-1,3-benzodioxoles. Aust. J. Chem. 1980, 33, 1553–1558. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).