5-((4-(-Phenyldiazenyl)phenyl)diazenyl)quinolin-8-ol
Abstract
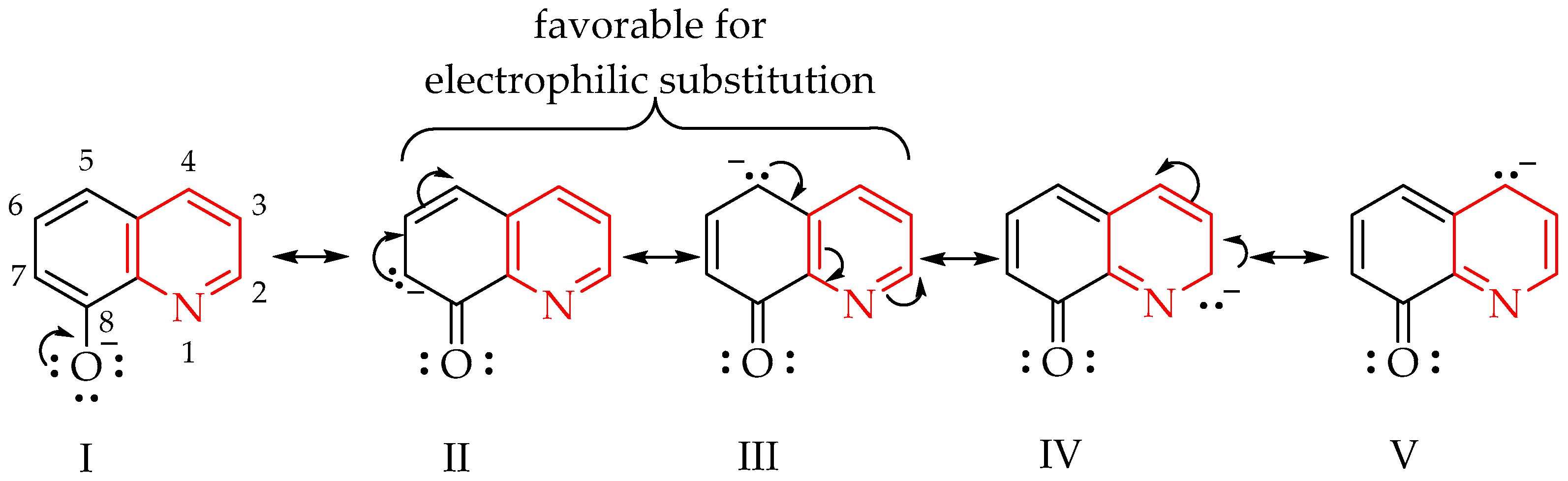
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Experimental Procedure
Synthesis of 5-(-(4-(-Phenyldiazenyl)phenyl)diazenyl)quinolin-8-ol (3)
- Diazonium Salt Synthesis
- Azo Coupling Reaction
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clark, M. Azoic dyeing. In Handbook of Textile and Industrial Dyeing; Woodhead Publishing Limited: Cambridge, UK, 2011; Volume 1, pp. 604–605. [Google Scholar]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo Dyes: Past, Present and the Future. Environ. Rev. 2011, 19, 350–371. [Google Scholar] [CrossRef]
- Di Martino, M.; Sessa, L.; Di Matteo, M.; Panunzi, B.; Piotto, S.; Concilio, S. Azobenzene as Antimicrobial Molecules. Molecules 2022, 27, 5643. [Google Scholar] [CrossRef] [PubMed]
- Bahrenburg, J.; Sievers, C.M.; Schönborn, J.B.; Hartke, B.; Renth, F.; Temps, F.; Näther, C.; Sönnichsen, F.D. Photochemical Properties of Multi-Azobenzene Compounds. Photochem. Photobiol. Sci. 2013, 12, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Crespi, S.; Simeth, N.A.; König, B. Heteroaryl Azo Dyes as Molecular Photoswitches. Nat. Rev. Chem. 2019, 3, 133–146. [Google Scholar] [CrossRef]
- Rashid, M.A.M.; Hayati, D.; Kwak, K.; Hong, J. Theoretical Investigation of Azobenzene-Based Photochromic Dyes for Dye-Sensitized Solar Cells. Nanomaterials 2020, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Ilina, K.; Henary, M. Cyanine Dyes Containing Quinoline Moieties: History, Synthesis, Optical Properties, and Applications. Chem. Eur. J. 2021, 27, 4230–4248. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Kim, S.-H.; Kim, Y.-S.; Kim, S.-H.; Son, Y.-A. Synthesis and Characterization of Quinoline-Based Dye Sensor. Mol. Cryst. 2009, 504, 173–180. [Google Scholar] [CrossRef]
- Czaplinska, B.; Maron, A.; Malecki, J.G.; Szafraniec-Gorol, G.; Matussek, M.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Danikiewicz, W.; Musiol, R.; Slodek, A. Comprehensive Exploration of the Optical and Biological Properties of New Quinoline Based Cellular Probes. Dye. Pigm. 2017, 144, 119–132. [Google Scholar] [CrossRef]
- Fahrni, C.J.; O’Halloran, T.V. Aqueous Coordination Chemistry of Quinoline-Based Fluorescence Probes for the Biological Chemistry of Zinc. J. Am. Chem. Soc. 1999, 121, 11448–11458. [Google Scholar] [CrossRef]
- Ali, R.R.; Mohammed, H.S. Biological activity and latent fingerprints detection by azo quinoline dye and its complexes. Period. Eng. Nat. Sci. 2021, 9, 317–329. [Google Scholar] [CrossRef]
- Witwit, I.; Yosif, Z.; Mubark, H. Synthesis, Characterization, and Biological Efficacy on New Mixed Ligand Complexes Based from Azo Dye of 8-Hydroxy Quinoline as a Primary Ligand and Imidazole as a Secondary Ligand with Some of Transition Metal Ions. J. Pharm. Sci. 2019, 11, 508–518. [Google Scholar]
- Moradi Rufchahi, E.O.; Pouramir, H.; Yazdanbakhsh, M.R.; Yousefi, H.; Bagheri, M.; Rassa, M. Novel Azo Dyes Derived from 8-Methyl-4-Hydroxyl-2-Quinolone: Synthesis, UV–Vis Studies and Biological Activity. Chin. Chem. Lett. 2013, 24, 425–428. [Google Scholar] [CrossRef]
- Butler, R.N. Diazotization of Heterocyclic Primary Amines. Chem. Rev. 1975, 75, 241–257. [Google Scholar] [CrossRef]
- Burcă, I.; Badea, V.; Deleanu, C.; Bercean, V.-N. 5-((8-Hydroxyquinolin-5-Yl)Diazenyl)-3-Methyl-1H-Pyrazole-4-Carboxylic Acid. Molbank 2021, 2021, M1238. [Google Scholar] [CrossRef]
- Phillips, J.P. The Reactions Of 8-Quinolinol. Chem. Rev. 1956, 56, 271–297. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.R.; Mahmoodi, N.O.; Dabiry, S. Preparation and Characterization of Diazenyl Quinolin-8-Ol with Trifluoromethyl Substituents. Mendeleev Commun. 2006, 16, 192–194. [Google Scholar] [CrossRef]
- Cipurković, A.; Horozić, E.; Marić, S.; Mekić, L.; Junuzović, H. Metal Complexes with 8-Hydroxyquinoline: Synthesis and In Vitro Antimicrobial Activity. Open J. Appl. Sci. 2021, 11, 106439. [Google Scholar] [CrossRef]
- Park, S.-Y.; Ghosh, P.; Park, S.O.; Lee, Y.M.; Kwak, S.K.; Kwon, O.-H. Origin of Ultraweak Fluorescence of 8-Hydroxyquinoline in Water: Photoinduced Ultrafast Proton Transfer. RSC Adv. 2016, 6, 9812–9821. [Google Scholar] [CrossRef]
- Savić-Gajić, I.M.; Savić, I.M. Drug Design Strategies with Metal-Hydroxyquinoline Complexes. Expert Opin. Drug Discov. 2020, 15, 383–390. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burcă, I.; Diaconescu, A.-M.; Badea, V.; Péter, F. 5-((4-(-Phenyldiazenyl)phenyl)diazenyl)quinolin-8-ol. Molbank 2023, 2023, M1701. https://doi.org/10.3390/M1701
Burcă I, Diaconescu A-M, Badea V, Péter F. 5-((4-(-Phenyldiazenyl)phenyl)diazenyl)quinolin-8-ol. Molbank. 2023; 2023(3):M1701. https://doi.org/10.3390/M1701
Chicago/Turabian StyleBurcă, Ion, Alexandra-Mihaela Diaconescu, Valentin Badea, and Francisc Péter. 2023. "5-((4-(-Phenyldiazenyl)phenyl)diazenyl)quinolin-8-ol" Molbank 2023, no. 3: M1701. https://doi.org/10.3390/M1701
APA StyleBurcă, I., Diaconescu, A.-M., Badea, V., & Péter, F. (2023). 5-((4-(-Phenyldiazenyl)phenyl)diazenyl)quinolin-8-ol. Molbank, 2023(3), M1701. https://doi.org/10.3390/M1701






