[μ-1,2-Bis(dipheylphosphino)ethane-κ2P,P’]bis(3-mercapto-1,2-propanediolato-κS-gold(I))
Abstract
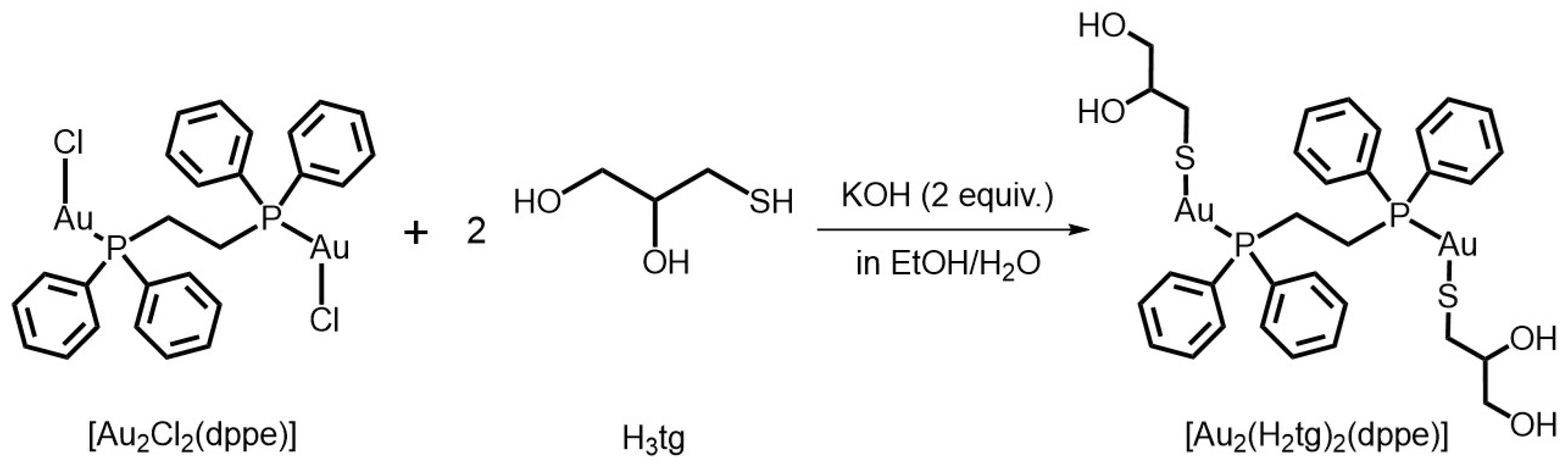
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of 1
2.2. Crystal Structure of 1
3. Materials and Methods
3.1. Materials
3.2. Physical Measurements
3.3. X-ray Crystal Structure Determination
3.4. Synthesis of [Au2(H2tg)2(dppe)] (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazuhara, A. A Raman spectroscopic investigation of the mechanism of the reduction in hair with thioglycerol and the accompanying disulphide conformational changes. Int. J. Cosmet. Sci. 2018, 40, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, W.K.; Dhariwal, G.; McKelvey, H.A.; McCabe, E.R.B. 1-thioglycerol: Inhibitor of glycerol kinase activity in vitro and in situ. Life Sci. 1986, 39, 1417–1424. [Google Scholar] [CrossRef]
- Vossmeyer, T.; Katsikas, L.; Giersig, M.; Popovic, I.G.; Chemseddine, A.; Eychmüller, A.; Weller, H. CdS Nanoclusters: Synthesis, Characterization, Size Dependent Oscillator Strength, Temperature Shift of the Excitonic Transition Energy, and Reversible Absorbance Shift. J. Phys. Chem. 1994, 98, 7665–7673. [Google Scholar] [CrossRef]
- Rogach, A.L.; Kornowski, A.; Gao, M.; Eychmüller, A. Horst Weller Synthesis and Characterization of a Size Series of Extremely Small Thiol-Stabilized CdSe Nanocrystals. J. Phys. Chem. B 1999, 103, 3065–3069. [Google Scholar] [CrossRef]
- Silva, A.C.A.; da Silva, S.W.; Morais, P.C.; Dantas, N.O. Shell Thickness Modulation in Ultrasmall CdSe/CdSxSe1–x/CdS Core/Shell Quantum Dots via 1-Thioglycerol. ACS Nano 2014, 8, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Rogach, A.L.; Katsikas, L.; Kornowski, A.; Su, D.; Eychmüller, H.A. Eychmüller; H. Weller Synthesis and characterization of thiol-stabilized CdTe nanocrystals. Berich. Bunsen. Gesell. 1996, 100, 1772–1778. [Google Scholar] [CrossRef]
- Dhobale, S.; Thite, T.; Laware, S.L.; Rode, C.V.; Koppikar, S.J.; Ghanekar, R.-K.; Kale, S.N. Zinc oxide nanoparticles as novel alpha-amylase inhibitors. J. Appl. Phys. 2008, 104, 094907. [Google Scholar] [CrossRef]
- Kumbhojkar, N.; Nikesh, V.V.; Kshirsagar, A.; Mahamuni, S. Photophysical properties of ZnS nanoclusters. J. Appl. Phys. 2000, 88, 6260–6264. [Google Scholar] [CrossRef]
- Kim, H.; Cho, K.; Song, H.; Min, B.; Lee, J.-S.; Kim, G.-T.; Kim, S.; Kim, S.H.; Noh, T. Photocurrent mechanism in a hybrid system of 1-thioglycerol-capped HgTe nanoparticles. J. Appl. Phys. 2003, 83, 4619–4621. [Google Scholar] [CrossRef]
- Kumaria, Y.; Jangir, L.K.; Kumar, A.; Kumara, M.; Awasthi, K. Investigation of thermal stability of TiO2 nanoparticles using 1-thioglycerol as capping agent. Solid State Commun. 2017, 263, 1–5. [Google Scholar] [CrossRef]
- Gaia, I.A.T.; Guimarães, E.V.; Maia, P.I.S.; Mikhail, H.D.; da Luz, M.S.; S, A.C.A.; Silva, R.S. Synthesis and investigation of optical and structural properties of Bi2O3/Bi2S3 nanoparticles in an aqueous solution. Physica B 2023, 662, 414947. [Google Scholar] [CrossRef]
- Chai, O.J.H.; Wu, Z.; Xie, J. All Hydroxyl-Thiol-Protected Gold Nanoclusters with Near-Neutral Surface Charge. J. Phys. Chem. Lett. 2021, 12, 9882–9887. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.M.; Cölfen, H. Formation of Nanoclusters in Gold Nucleation. Crystals 2020, 10, 382. [Google Scholar] [CrossRef]
- Yao, H.; Yaomura, S. Emergence of Large Chiroptical Responses by Ligand Exchange Cross-Linking of Monolayer-Protected Gold Clusters with Chiral Dithiol. Langmuir 2013, 29, 6444–6451. [Google Scholar] [CrossRef] [PubMed]
- Sumi, T.; Motono, S.; Ishida, Y.; Shirahata, N.; Yonezawa, T. Formation and Optical Properties of Fluorescent Gold Nanoparticles Obtained by Matrix Sputtering Method with Volatile Mercaptan Molecules in the Vacuum Chamber and Consideration of Their Structures. Langmuir 2015, 31, 4323–4329. [Google Scholar] [CrossRef] [PubMed]
- Komarov, V.P.; Lazarev, V.B.; Shaplygin, I.S. Preparation and thermal decomposition of complexes of silver(I) and gold(I) with thiovanol. Zhurnal Neorg. Khimii 1980, 25, 746–751. [Google Scholar]
- Nigam, H.L.; Kumar, A.N.; Pandeya, K.B. Infrared absorption spectra of metal complexes of some sulfur containing ligands. Proc. Chem. Symp. 1970, 2, 53–57. [Google Scholar]
- Hager, C.-D.; Huber, F. (Hydroxyalkyl)- und (4-Hydroxyphenyl)organoplumbylsulfide. Z. Naturforsch. 1980, 35, 931–933. [Google Scholar] [CrossRef]
- Vossmeyer, T.; Reck, G.; Katsikas, L.; Haupt, E.T.K.; Schulz, B.; Weller, H. A New Three Dimensional Crystal Structure of a Cadmium Thiolate. Inorg. Chem. 1995, 34, 4926–4929. [Google Scholar] [CrossRef]
- López-de-Luzuriaga, J.M.; Sladek, A.; Schmidbaur, H. Mixed coordination numbers and geometries of gold(I) in a dinuclear complex of thioglycerol. J. Chem. Soc. Dalton Trans. 1996, 4511–4512. [Google Scholar] [CrossRef]
- Tekeste, T.; Vahrenkamp, H. Modeling zinc enzyme inhibition with functional thiolate ligands. Inorg. Chem. 2006, 45, 10799–10806. [Google Scholar] [CrossRef] [PubMed]
- Kyprianidou, E.J.; Lazarides, T.; Kaziannis, S.; Kosmidis, C.; Itskos, G.; Manos, M.J.; Tasiopoulos, A.J. Single crystal coordinating solvent exchange as a general method for the enhancement of the photoluminescence properties of lanthanide MOFs. J. Mater. Chem. A 2014, 2, 5258–5266. [Google Scholar] [CrossRef]
- Manos, M.J.; Kyprianidou, E.J.; Papaefstathiou, G.S.; Tasiopoulos, A.J. Insertion of Functional Groups into a Nd3+ Metal–Organic Framework via Single-Crystal-to-Single-Crystal Coordinating Solvent Exchange. Inorg. Chem. 2012, 51, 6308–6314. [Google Scholar] [CrossRef] [PubMed]
- Dance, I.G. The structural chemistry of metal thiolate complexes. Polyhedron 1986, 5, 1037–1104. [Google Scholar] [CrossRef]
- Yoshinari, N.; Konno, T. Metallosupramolecular Structures Derived from a Series of Diphosphine-bridged Digold(I) Metalloligands with Terminal d-Penicillamine. Chem. Rec. 2016, 16, 1647–1663. [Google Scholar] [CrossRef]
- Pyykkö, P. Strong Closed-Shell Interactions in Inorganic Chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. A briefing on aurophilicity. Chem. Soc. Rev. 2008, 37, 1931–1951. [Google Scholar] [CrossRef]
- Mirabelli, C.K.; Hill, D.T.; Faucette, L.F.; McCabe, F.L.; Girard, G.R.; Bryan, D.B.; Sutton, B.M.; Bartus, J.O.; Crooke, S.T.; Johnson, R.K. Antitumor Activity of Bis(diphenylphosphino)alkanes, Their Gold(I) Coordination Complexes, and Related Compounds. J. Med. Chem. 1987, 30, 2181–2190. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristic Group Frequencies, 3rd ed.; Wiley: Chichester, UK, 2001. [Google Scholar]
- Sabot, C.; Kumar, K.A.; Antheaume, C.; Mioskowski, C. Triazabicyclodecene: An Effective Isotope Exchange Catalyst in CDCl3. J. Org. Chem. 2007, 72, 5001–5004. [Google Scholar] [CrossRef]
- Tzeng, B.-C.; Liao, J.-H.; Lee, G.-H.; Peng, S.-M. Photophysical properties, electronic and crystal structures of luminescent diphosphine digold(I)-pyridine-2-thiolate complexes. Inorg. Chim. Acta 2004, 357, 1405–1410. [Google Scholar] [CrossRef]
- Li, C.-K.; Lu, X.-X.; Wong, K.M.-C.; Chan, C.-L.; Zhu, N.; Yam, V.W.-W. Molecular Design of Luminescence Ion Probes for Various Cations Based on Weak Gold(I)···Gold(I) Interactions in Dinuclear Gold(I) Complexes. Inorg. Chem. 2004, 43, 7421–7430. [Google Scholar] [CrossRef] [PubMed]
- Bardají, M.; Calhorda, M.J.; Costa, P.J.; Jones, P.G.; Laguna, A.; Pérez, M.R.; Villacampa, M.D. Synthesis, Structural Characterization, and Theoretical Studies of Gold(I) and Gold(I)−Gold(III) Thiolate Complexes: Quenching of Gold(I) Thiolate Luminescence. Inorg. Chem. 2006, 45, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.J.; Calhorda, M.J. A DFT and MP2 study of luminescence of gold(I) complexes. Inorg. Chim. Acta 2006, 359, 3617–3624. [Google Scholar] [CrossRef]
- Schneider, J.; Lee, Y.-A.; Pérez, J.; Brennessel, W.W.; Flaschenriem, C.; Eisenberg, R. Strong Intra- and Intermolecular Aurophilic Interactions in a New Series of Brilliantly Luminescent Dinuclear Cationic and Neutral Au(I) Benzimidazolethiolate Complexes. Inorg. Chem. 2008, 47, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Tiekink, E.R.T.; Kang, J.-G. Luminescence properties of phosphinegold(I) halides and thiolates. Coord. Chem. Rev. 2009, 253, 1627–1648. [Google Scholar] [CrossRef]
- Koshevoy, I.O.; Smirnova, E.S.; Haukka, M.; Laguna, A.; Chueca, J.C.; Pakkanen, T.A.; Tunik, S.P.; Ospino, I.; Crespo, O. Synthesis, structural characterization, photophysical properties and theoretical analysis of gold(I) thiolate-phosphine complexes. Dalton Trans. 2011, 40, 7412–7422. [Google Scholar] [CrossRef]
- Crespo, O.; Gimeno, M.C.; Laguna, A.; Lahoz, F.J.; Larraz, C. Unprecedented Luminescent Heteropolynuclear Aggregates with Gold Thiolates as Building Blocks. Inorg. Chem. 2011, 50, 9533–9544. [Google Scholar] [CrossRef]
- Moreno-Alcántar, G.; Romo-Islas, G.; Flores-Álamo, M.; Torrens, H. Aurophilicity vs. thiophilicity: Directing the crystalline supramolecular arrangement in luminescent gold compounds. New J. Chem. 2018, 42, 7845–7852. [Google Scholar] [CrossRef]
- Onaka, S.; Yaguchi, M.; Yamauchi, R.; Ozeki, T.; Ito, M.; Sunahara, T.; Sugiura, Y.; Shiotsuka, M.; Nunokawa, K.; Horibe, M.; et al. The effect of carbon chain length of the diphosphine ligand on the aurophilic interaction. Synthesis and X-ray structural study for a series of Au(I) compounds with Ph2P–R–PPh2 and S-(CH2)n-py ligands. J. Organomet. Chem. 2005, 690, 57–68. [Google Scholar] [CrossRef]
- Artigas, M.M.; Crespo, O.; Gimeno, M.C.; Jones, P.G.; Laguna, A.; Villacampa, M.D. Gold(I) complexes with the ligand 1-thiolate-1,2-dicarba-closo-dodecaborate. Crystal structure of [Au2(1-S-1,2-C2B10H11)2(μ-dppe)]. J. Organomet. Chem. 1998, 561, 1–6. [Google Scholar] [CrossRef]
- Ho, S.Y.; Cheng, E.C.-C.; Tiekink, E.R.T.; Yam, V.W.-W. Luminescent Phosphine Gold(I) Thiolates: Correlation between Crystal Structure and Photoluminescent Properties in [R3PAu{SC(OMe)=NC6H4NO2-4}] (R = Et, Cy, Ph) and [(Ph2P-R-PPh2){AuSC(OMe)=NC6H4NO2-4}2] (R = CH2, (CH2)2, (CH2)3, (CH2)4, Fc). Inorg. Chem. 2006, 45, 8165–8174. [Google Scholar] [CrossRef] [PubMed]
- Ilie, A.; Raţ, C.I.; Scheutzow, S.; Kiske, C.; Lux, K.; Klapötke, T.M.; Silvestru, C.; Karaghiosoff, K. Metallophilic Bonding and Agostic Interactions in Gold(I) and Silver(I) Complexes Bearing a Thiotetrazole Unit. Inorg. Chem. 2011, 50, 2675–2684. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Takemoto, M.; Osaka, K.; Nishibori, E.; Moriyoshi, C.; Kubota, Y.; Kuroiwa, Y.; Sugimoto, K. High-throughput powder diffraction measurement system consisting of multiple MYTHEN detectors at beamline BL02B2 of SPring-8. Rev. Sci. Instrum. 2017, 88, 085111. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.N. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Yoshinari, N.; Konno, T. Chiral Phenomena in Multinuclear and Metallosupramolecular Coordination Systems Derived from Metalloligands with Thiol-containing Amino Acids. Bull. Chem. Soc. Jpn. 2018, 91, 790–812. [Google Scholar] [CrossRef]
- Yoshinari, N.; Kuwamura, N.; Kojima, T.; Konno, T. Development of Coordination Chemistry with Thiol-containing Amino Acids. Coord. Chem. Rev. 2023, 474, 214857. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baba, T.; Yoshinari, N. [μ-1,2-Bis(dipheylphosphino)ethane-κ2P,P’]bis(3-mercapto-1,2-propanediolato-κS-gold(I)). Molbank 2023, 2023, M1698. https://doi.org/10.3390/M1698
Baba T, Yoshinari N. [μ-1,2-Bis(dipheylphosphino)ethane-κ2P,P’]bis(3-mercapto-1,2-propanediolato-κS-gold(I)). Molbank. 2023; 2023(3):M1698. https://doi.org/10.3390/M1698
Chicago/Turabian StyleBaba, Taichi, and Nobuto Yoshinari. 2023. "[μ-1,2-Bis(dipheylphosphino)ethane-κ2P,P’]bis(3-mercapto-1,2-propanediolato-κS-gold(I))" Molbank 2023, no. 3: M1698. https://doi.org/10.3390/M1698
APA StyleBaba, T., & Yoshinari, N. (2023). [μ-1,2-Bis(dipheylphosphino)ethane-κ2P,P’]bis(3-mercapto-1,2-propanediolato-κS-gold(I)). Molbank, 2023(3), M1698. https://doi.org/10.3390/M1698





