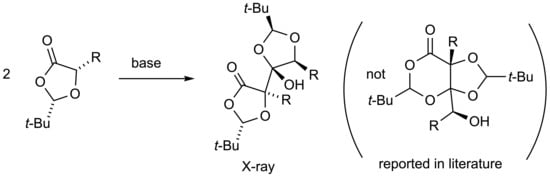
(2S,2’S,4R,5S,5’R)-2,2’-Di-tert-butyl-4-hydroxy-5,5’-dimethyl-4,5’-bi(1,3-dioxolanyl)-4’-one
Abstract
1. Introduction
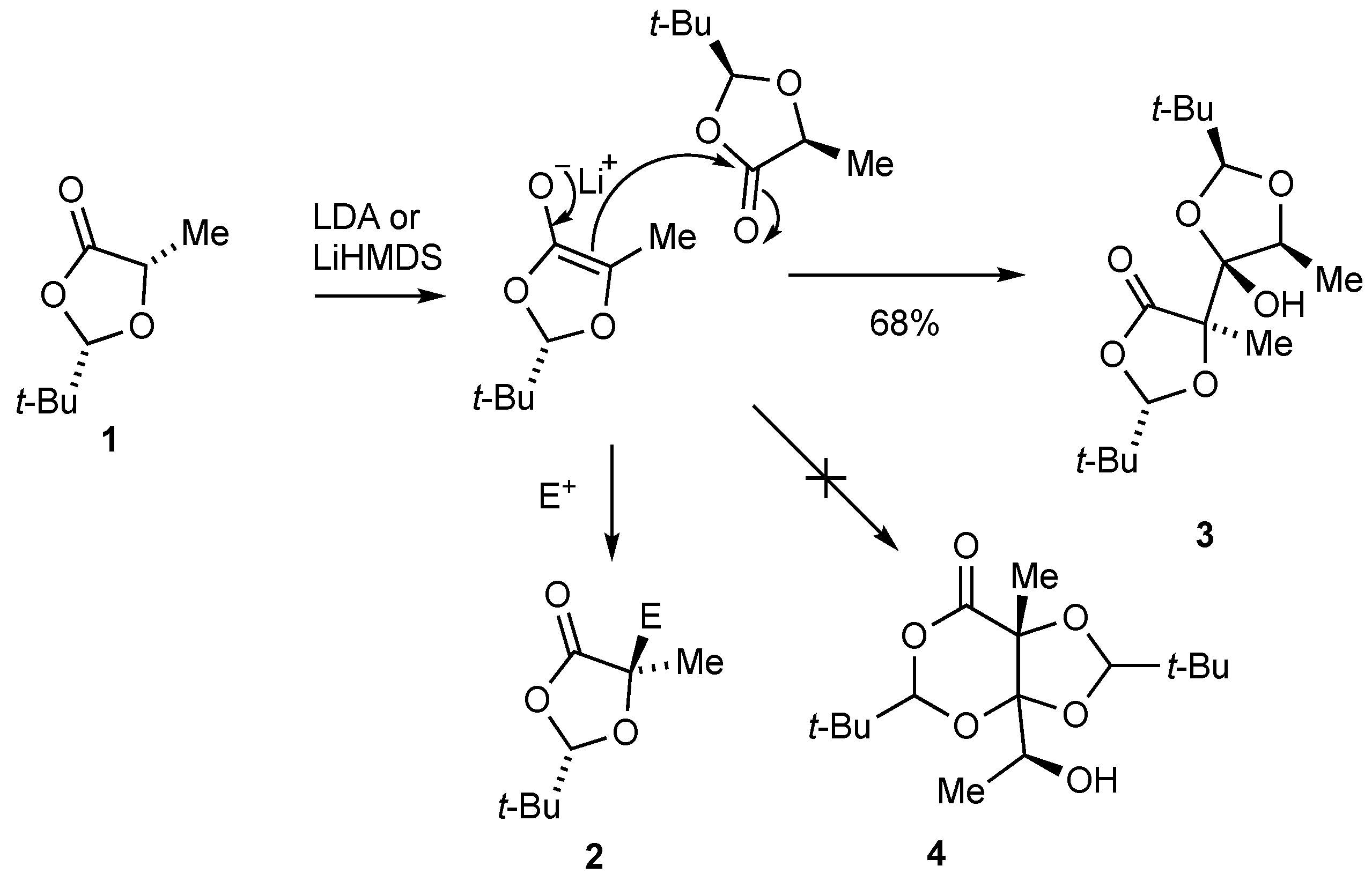
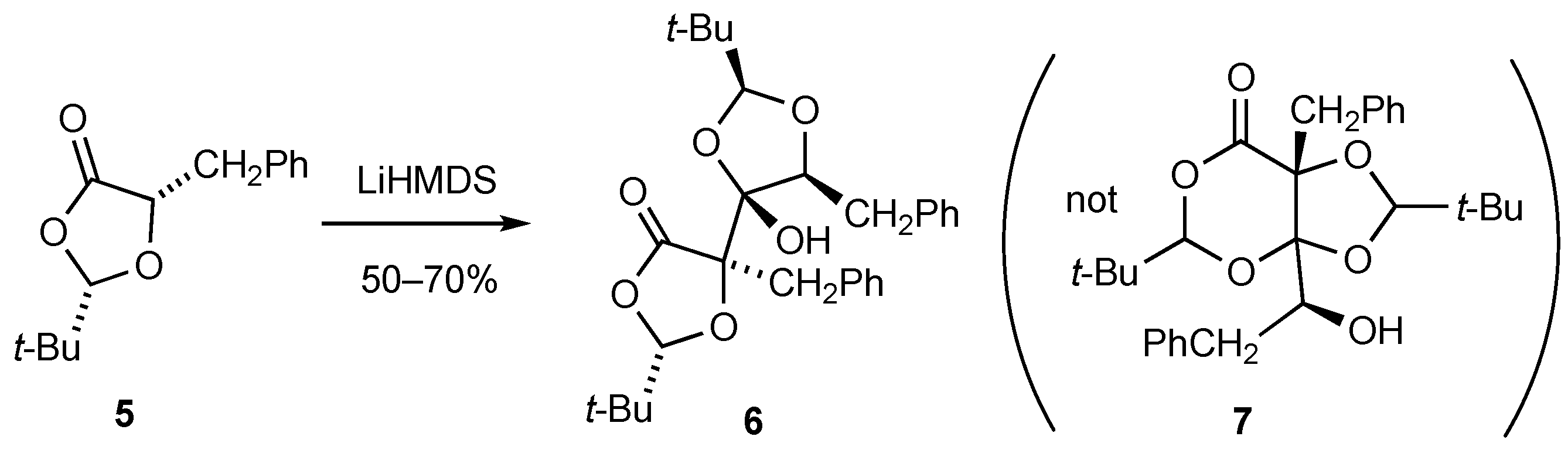
2. Results and Discussion
3. Experimental
3.1. Formation of (2S,2’S,4R,5S,5’R)-2,2’-Di-tert-butyl-4-hydroxy-5,5’-dimethyl-4,5’-bi(1,3-dioxolanyl)-4’-one 3
3.2. X-ray Structure Determination of 3
3.3. Formation of (2S,2’S,4’R,5S,5’R)-5,5’-Dibenzyl-2,2’-di-tert-butyl-4-hydroxy-4,5’-bi [1,3-dioxolanyl]-4’-one 6
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seebach, D.; Naef, R. Enantioselective generation and diastereoselective reactions of chiral enolates derived from α-heterosubstituted carboxylic acids. Preliminary Communication. Helv. Chim. Acta 1981, 64, 2704–2708. [Google Scholar] [CrossRef]
- Seebach, D.; Naef, R.; Calderari, G. α-Alkylation of α-heterosubstituted carboxylic acids without racemization: EPC-synthesis of tertiary alcohols and thiols. Tetrahedron 1984, 40, 1313–1324. [Google Scholar] [CrossRef]
- Barbaro, G.; Battaglia, A.; Guerrini, A.; Bertucci, C. One-pot synthesis of (3R)-hydroxy-β-lactams via enolates of 2-tert-butyl-1,3-dioxolan-4-ones. Part 1. Tetrahedron Asymmetry 1997, 8, 2527–2531. [Google Scholar] [CrossRef]
- Aitken, R.A.; Thomas, A.W. Behaviour of dioxolanones as chiral acyl anion equivalents. Synlett 1998, 1998, 102–104. [Google Scholar] [CrossRef]
- Aitken, R.A.; Power, L.A.; Slawin, A.M.Z. New chemistry of chiral 1,3-dioxolan-4-ones. Molecules 2023, 28, 3845. [Google Scholar] [CrossRef] [PubMed]
- Calderari, G.; Seebach, D. Asymmetrische Michael-Additionen. Stereoselektive Alkylierung chiraler, nicht racemischer Enolate durch Nitroolefine. Herstellung enantiomerenreiner γ-Aminobuttersäure- und Bernsteinsäure-Derivate. Helv. Chim. Acta 1985, 68, 1592–1604. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B 1990, 46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]

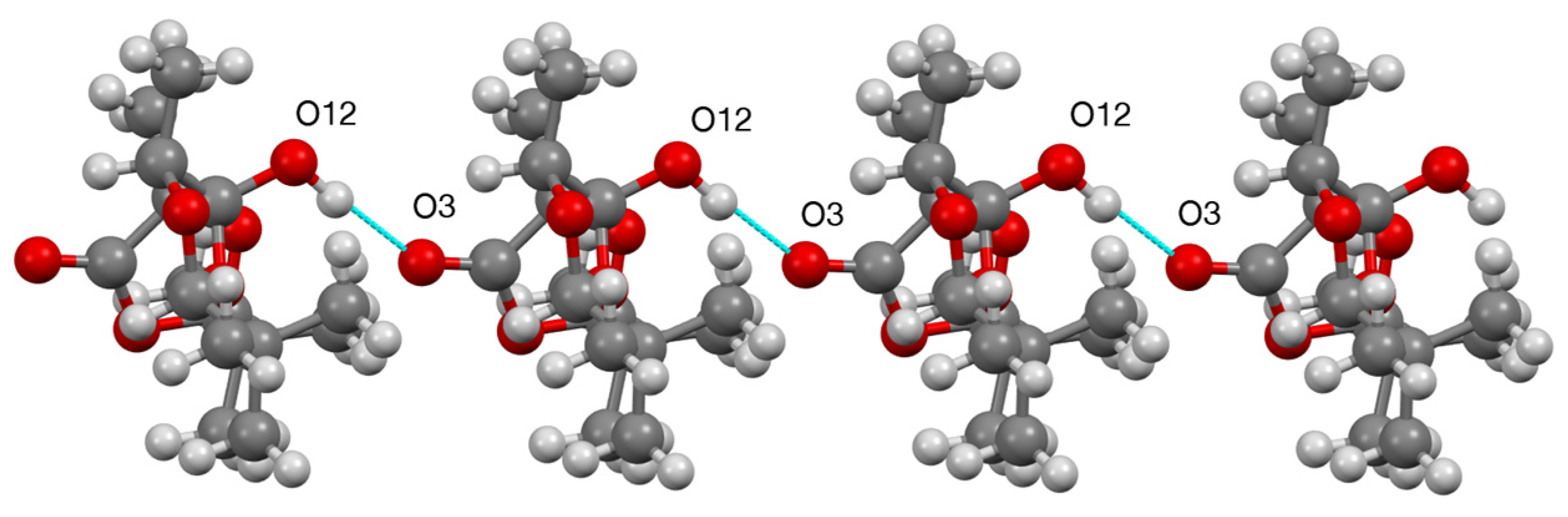
| D—H…A | D—H | H…A | D…A | D—H…A |
|---|---|---|---|---|
| O(12)–H(12)…O(3) | 0.98 | 1.88 | 2.853(10) | 174.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Power, L.A.; Slawin, A.M.Z. (2S,2’S,4R,5S,5’R)-2,2’-Di-tert-butyl-4-hydroxy-5,5’-dimethyl-4,5’-bi(1,3-dioxolanyl)-4’-one. Molbank 2023, 2023, M1699. https://doi.org/10.3390/M1699
Aitken RA, Power LA, Slawin AMZ. (2S,2’S,4R,5S,5’R)-2,2’-Di-tert-butyl-4-hydroxy-5,5’-dimethyl-4,5’-bi(1,3-dioxolanyl)-4’-one. Molbank. 2023; 2023(3):M1699. https://doi.org/10.3390/M1699
Chicago/Turabian StyleAitken, R. Alan, Lynn A. Power, and Alexandra M. Z. Slawin. 2023. "(2S,2’S,4R,5S,5’R)-2,2’-Di-tert-butyl-4-hydroxy-5,5’-dimethyl-4,5’-bi(1,3-dioxolanyl)-4’-one" Molbank 2023, no. 3: M1699. https://doi.org/10.3390/M1699
APA StyleAitken, R. A., Power, L. A., & Slawin, A. M. Z. (2023). (2S,2’S,4R,5S,5’R)-2,2’-Di-tert-butyl-4-hydroxy-5,5’-dimethyl-4,5’-bi(1,3-dioxolanyl)-4’-one. Molbank, 2023(3), M1699. https://doi.org/10.3390/M1699