Abstract
The preparation and characterization of a new multi-functionalized terpyridine molecule featuring a pyrrole heterocycle and a cyano group is described. This new compound was obtained via a KF/alumina-catalyzed Michael addition of 4′-(pyrrol-2-yl)-2,2′:6′,2″-terpyridine into acrylonitrile. The mild reaction conditions leave the nitrile group unaltered. The title compound was fully characterized via NMR spectroscopy (1H and 13C) as well as via high resolution mass spectrometry and infrared spectroscopy.
1. Introduction
Terpyridines and their metal complexes are molecules that find a broad range of applications in many fields [1,2]. Especially interesting are terpyridine molecules, which feature an additional five-membered heterocycle, such as pyrrole (named terpyridine–pyrrole thereafter). In fact, the latter allows for the fabrication of modified electrodes via the electrochemical polymerization of terpyridine–metal complexes as thin films [3]. The thus obtained electrodes can be utilized as sensors [4]. Therefore, the preparation of new terpyridine–pyrrole derivatives is of interest, especially ones which are N-functionalized to the pyrrole moiety, as such derivatives may potentially be useful for the preparation of new functionalized thin films materials, for example.
There are some examples of terpyridine–pyrroles which are modified to the N-atom of the pyrrole unit [5,6,7]. To date, they are prepared via two synthetic strategies (Figure 1):
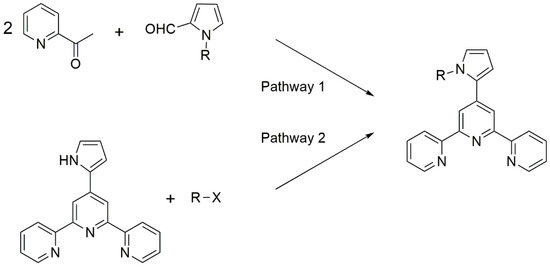
Figure 1.
Current synthetic strategies to prepare terpyridine–pyrrole derivatives functionalized to pyrrole nitrogen atom.
- The construction of the terpyridine system from an adequately N-functionalized pyrrole aldehyde.
- The N-alkylation of the pyrrole onto 4′-(pyrrol-2-yl)-2,2′:6′,2″-terpyridine.
Nevertheless, these synthetic pathways require basic media, which can result in side-reactions with certain substrates, as already reported in the literature [8,9]. Consequently, other synthetic protocols must be envisioned for the preparation of such terpyridine–pyrrole compounds.
This paper describes the preparation of the new terpyridine molecule 1 (Figure 2), which features a nitrile group. Molecule 1 was prepared through an aza-Michael addition of the pyrrole ring onto acrylonitrile. This reaction is catalyzed by potassium fluoride to alumina under mild conditions. The new ligand 1 was characterized by different analytical techniques, such as proton and carbon NMR, mass spectrometry and infrared spectroscopy.
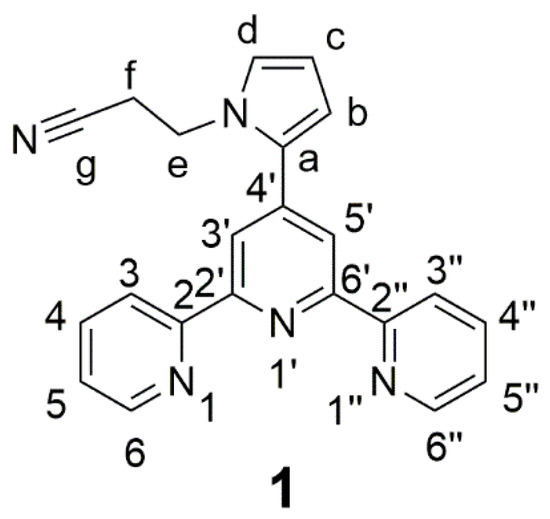
Figure 2.
Structure and atom labeling of compound 1.
2. Results and Discussion
2.1. Synthesis
The title compound was prepared through the reaction of 4′-(pyrrol-2-yl)-2,2′:6′,2″-terpyridine [6,10] with acrylonitrile, according to a protocol that has previously been used for the preparation of pyrrole derivatives [11]. It relies on an aza-Michael addition reaction, which is catalyzed via potassium fluoride to alumina (KF/alumina) and is performed at room temperature in dimethylformamide as solvent (Figure 3). After 50 h, no further reaction was noticed, and the product was separated from the starting material via flash chromatography over neutral alumina.
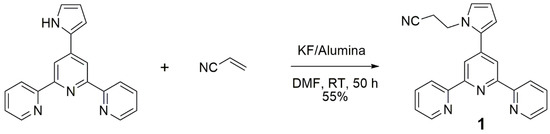
Figure 3.
Reaction scheme.
These mild reaction conditions tolerate the presence of the nitrile group, which remains unaltered, while attempts to prepare cyano-containing terpyridines from pathways 1 or 2 resulted in complex mixtures with many by-products.
2.2. Characterization
The structure of the product was confirmed via 1H and 13C NMR, attenuated total reflectance infrared spectroscopy (ATR-IR) and high-resolution mass spectrometry (HR-MS). The proton spectrum (Supplementary Materials) exhibits characteristic signals for terpyridine–pyrrole compounds. Furthermore, the aliphatic part of the molecule (protons He and Hf) appears as two triplets as expected. Additionally, the 13C NMR spectrum exhibits 15 peaks, as anticipated for compound 1.
The infrared spectrum recorded in the attenuated total reflectance (ATR) mode features the characteristic C≡N stretching vibration at 2252 cm−1.
Finally, the structure was further confirmed via HR-MS, which indicates a measured m/z of 424.16521 and is coherent with the calculated mass for the molecular ion [C26H21N3O3 + H]+ (m/z = 424.16557).
3. Materials and Methods
All reagents were purchased from commercial suppliers and used as received. 4′-(Pyrrol-2-yl)-2,2′:6′,2″-terpyridine was prepared according to the literature [6]. Flash chromatography was carried out on a Combiflash Rf + Lumen (Teledyne ISCO, Lincoln, NE, USA) using a CHROMABOND RS 40 Alox N 80 g neutral alumina cartridge (Macherey-Nagel, Düren, Germany) with a hexane/ethyl acetate mixture (100:0 to 90:10 v:v) as an eluent. 1H and 13C NMR spectra were recorded on a Brucker AC 400 (Bruker, Wissembourg, France) at 400 and 100 MHz, respectively, using CDCl3 as a solvent. Infrared spectrum was recorded on a Vertex 70 spectrometer (Bruker, Wissembourg, France) in ATR mode. The melting point was recorded with a M 565 melting point apparatus (Büchi, Flawil, Switzerland) according to European Pharmacopoeia. HR-MS was recorded at Sayence SATT, Dijon, France.
4′-(N-(2-cyanoethyl)pyrrol-2-yl)-2,2′:6′,2″-terpyridine: To a solution of 4′-(pyrrol-2-yl)-2,2′:6′,2″-terpyridine (1.49 g; 5.0 mmol) in DMF (10 mL), acrylonitrile (0.27 g; 5.1 mmol) and KF/alumina (0.090 g) were successively added. The resulting suspension was stirred at room temperature for 50 h. The mixture was diluted with ethyl acetate (100 mL). The organic phase was washed with water (3 × 100 mL) and brine (100 mL), dried over sodium sulfate and concentrated under reduced pressure. The crude product was purified via flash chromatography over neutral alumina to afford pure 1 as a beige solid (0.96 g; 55%) mp = 144.3 °C. 1H-NMR (CDCl3, 400 MHz), δ (ppm): 8.74 (ddd, 2H, H6, 6″, J = 4.8 Hz, J = 1.7 Hz, J = 0.8 Hz), 8.65 (d, 2H, H3, 3″), 8.49 (s, 2H, H3′,5′), 7.87 (td, 2H, H4, 4″, J = 7.7 Hz, J = 1.8 Hz), 7.36 (ddd, 2H, H5, 5″, J = 7.5 Hz, J = 4.8 Hz, J = 1.2 Hz), 6.91 (dd, 1H, Hd, J = 2.6 Hz, J = 1.8 Hz), 6.60 (dd, 1H, Hb, J = 3.7 Hz, J = 1.7 Hz), 6.28 (dd, 1H, Hc, J = 3.6 Hz, J = 2.8 Hz), 4.47 (t, 2H, Hf, J = 6.8 Hz), 2.66 (t, 2H, He, J = 6.8 Hz). 13C-NMR (CDCl3, 100 MHz), δ (ppm): 156.0, 155.9, 149.2, 141.9, 136.9, 131.6, 124.7, 124.0, 121.3, 119.5, 116.9, 112.8, 110.0, 43.4, 20.3. HR-MS: calc. for [C22H17N5 + H]+ 352.15567, found 352.15585. IR (ATR): νmax (cm−1): 3097.5, 3051.0, 3011.4, 2954.2, 2931.1, 2251.7.
4. Conclusions
The new oligopyridine ligand 4′-(N-(2-cyanoethyl)pyrrol-2-yl)-2,2′:6′,2″-terpyridine was prepared and characterized. The synthetic protocol that was used tolerated the base-sensitive nitrile group. This is a new tool to prepare new ligands that could be utilized to construct functionalized materials. Experiments are currently carried out to extend this methodology to the synthesis of new terpyridines by using this reaction with other Michael acceptors (acrylates, methacrylates) and by converting the nitrile group into other functional groups (e.g., amine, ketone). Results will be reported in due course.
Supplementary Materials
The following supporting information can be downloaded online: 1H, 13C and ATR-IR spectra; HR-MS full report and melting point report.
Funding
This research was funded by the Région Bourgogne Franche-Comté (FINEAU Project) and by OSU THETA (BQR 2022).
Data Availability Statement
The data from this study are available in this paper and are presented in the Supplementary Materials.
Conflicts of Interest
The author declares no conflict of interest.
References
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Schubert, U.S.; Winter, A.; Newkome, G.R. Terpyridine-Based Materials: For Catalytic, Optoelectronic and Life Sciences Applications; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Friebe, C.; Hager, M.H.; Winter, A.; Schubert, U.S. Metal-containing Polymers via Electropolymerization. Adv. Mater. 2012, 24, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Naidji, B.; Husson, J.; Taouil, A.E.; Brunol, E.; Sanchez, J.-B.; Berger, F.; Rauch, J.-Y.; Guyard, L. Terpyridine-based metallopolymer thin films as active layer in ammonia sensor device. Synth. Met. 2016, 221, 214–219. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. A new and facile method for the functionalization of a Merrifield resin with terpyridines: Application as a heterogeneous catalyst for the synthesis of biaryls in environmentally friendly solvents. Green Process. Synth. 2016, 5, 331–336. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. Synthesis of new 4′-(N-alkylpyrrol-2-yl)-2,2′:6′,2″-terpyridines via N-alkylation of a pyrrole moiety. Heterocycl. Commun. 2015, 21, 199–202. [Google Scholar] [CrossRef]
- Klemens, T.; Switlicka-Olszewska, A.; Machura, B.; Grucela, M.; Schab-Balcerzak, E.; Smolarek, K.; Mackowski, S.; Szlapa, A.; Kula, S.; Krompiec, S.; et al. Rhenium(I) terpyridine complexes—Synthesis, photophysical properties and application in organic light emitting devices. Dalton Trans. 2016, 45, 1746–1762. [Google Scholar] [CrossRef] [PubMed]
- Husson, J.; Guyard, L. 4′-(N-(propan-1,2-dienyl)pyrrol-2-yl)-2,2′:6′,2″-terpyridine. Molbank 2020, 2020, M1142. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. 4′-(N-(propargyl)pyrrol-2-yl)-2,2′:6′,2″-terpyridine. Molbank 2022, 2022, M1356. [Google Scholar] [CrossRef]
- Abboud, M.; Kalinina, D.; Potvin, P.G. Pyrrole-substituted tridentate complexes of Ru(II): Spectroscopy, electrochemistry, photosensitization and the role of orbital mixing. Inorg. Chim. Acta 2009, 362, 4953–4959. [Google Scholar] [CrossRef]
- Yang, L.; Xu, L.-W.; Xia, C.-G. Highly efficient KF/Al2O3-catalyzed versatile hetero-Michael addition of nitrogen, oxygen, and sulfur nucleophiles to α,β-ethylenic compounds. Tetrahedron Lett. 2005, 46, 3279–3282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).