N,N′-Bis(3-ethoxy-2-hydroxybenzylidene)-phenylene-1,3-diamine Methanol Solvate
Abstract
1. Introduction
2. Results and Discussion
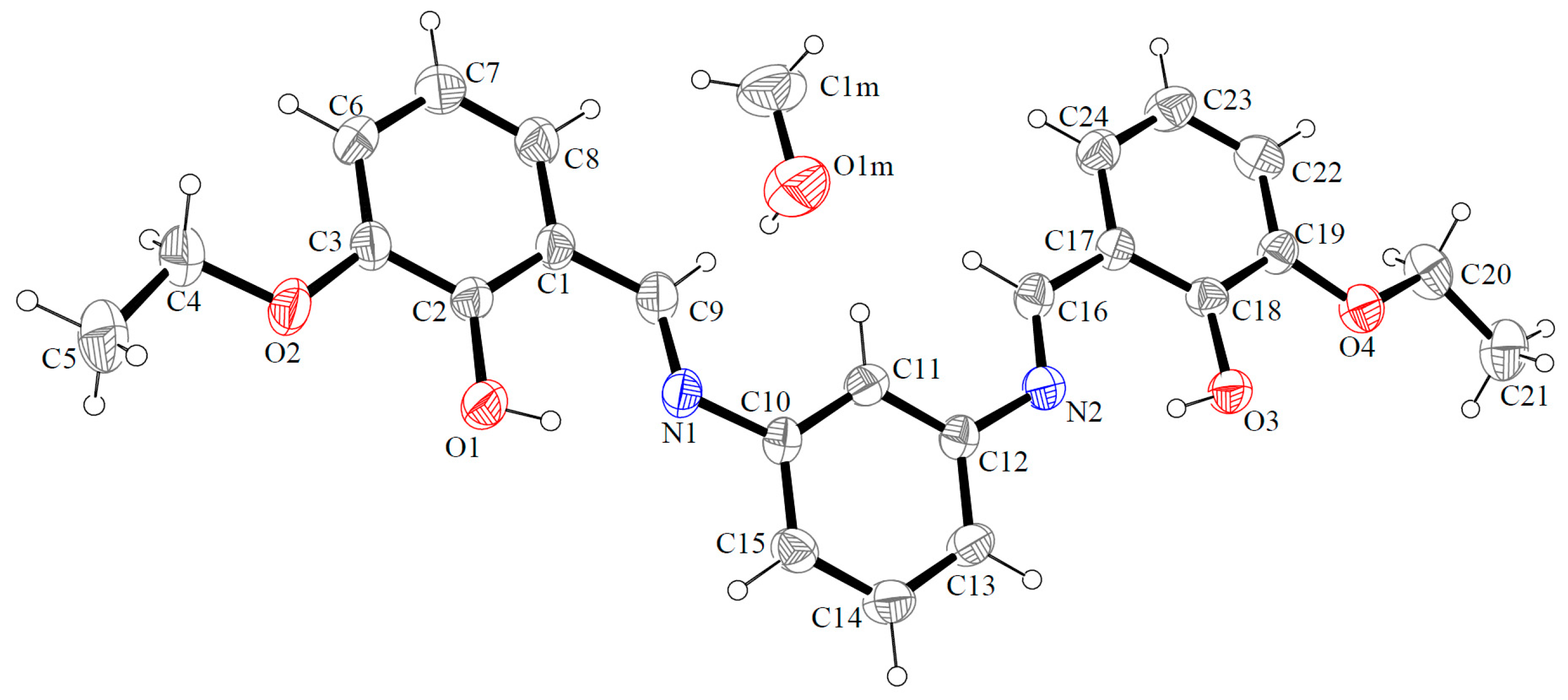
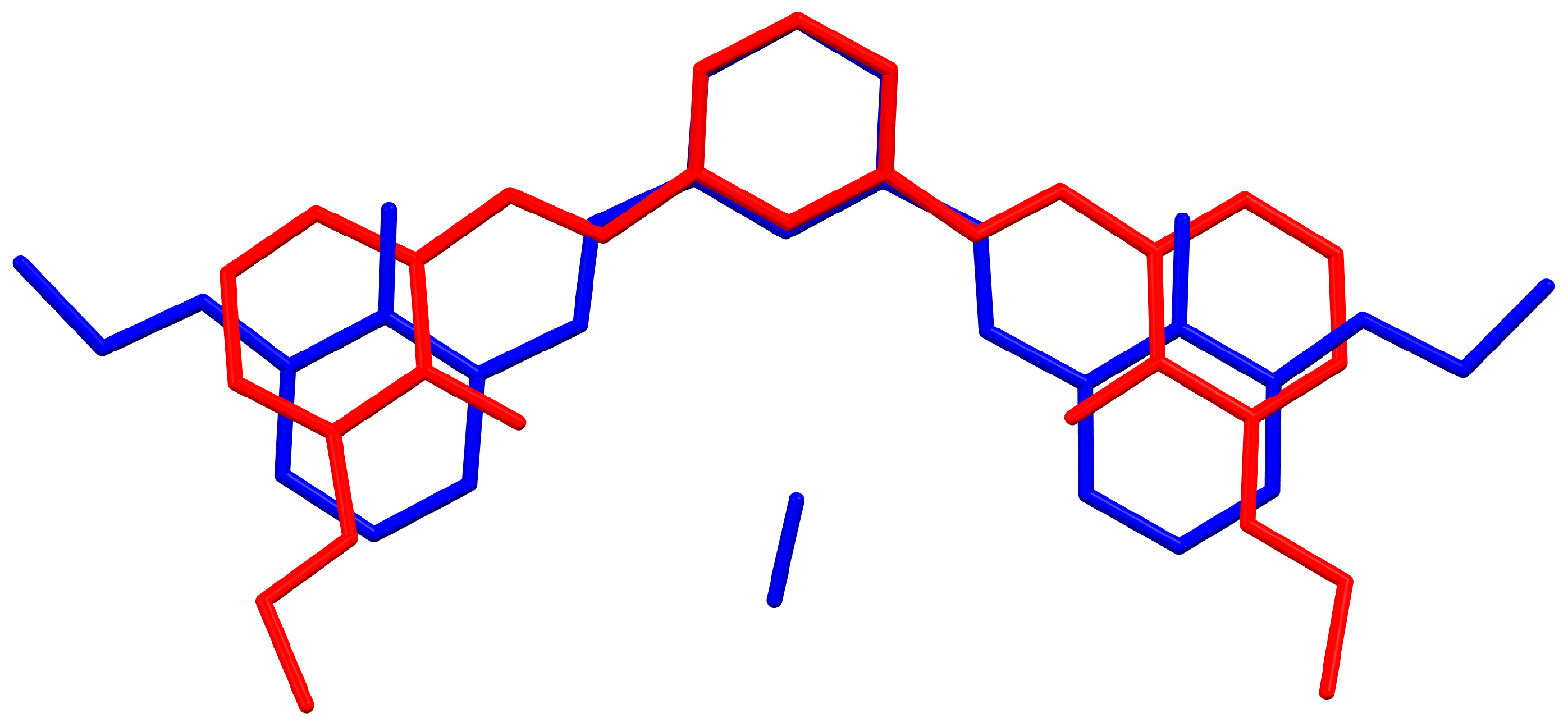
2.1. Crystal and Molecular Structure
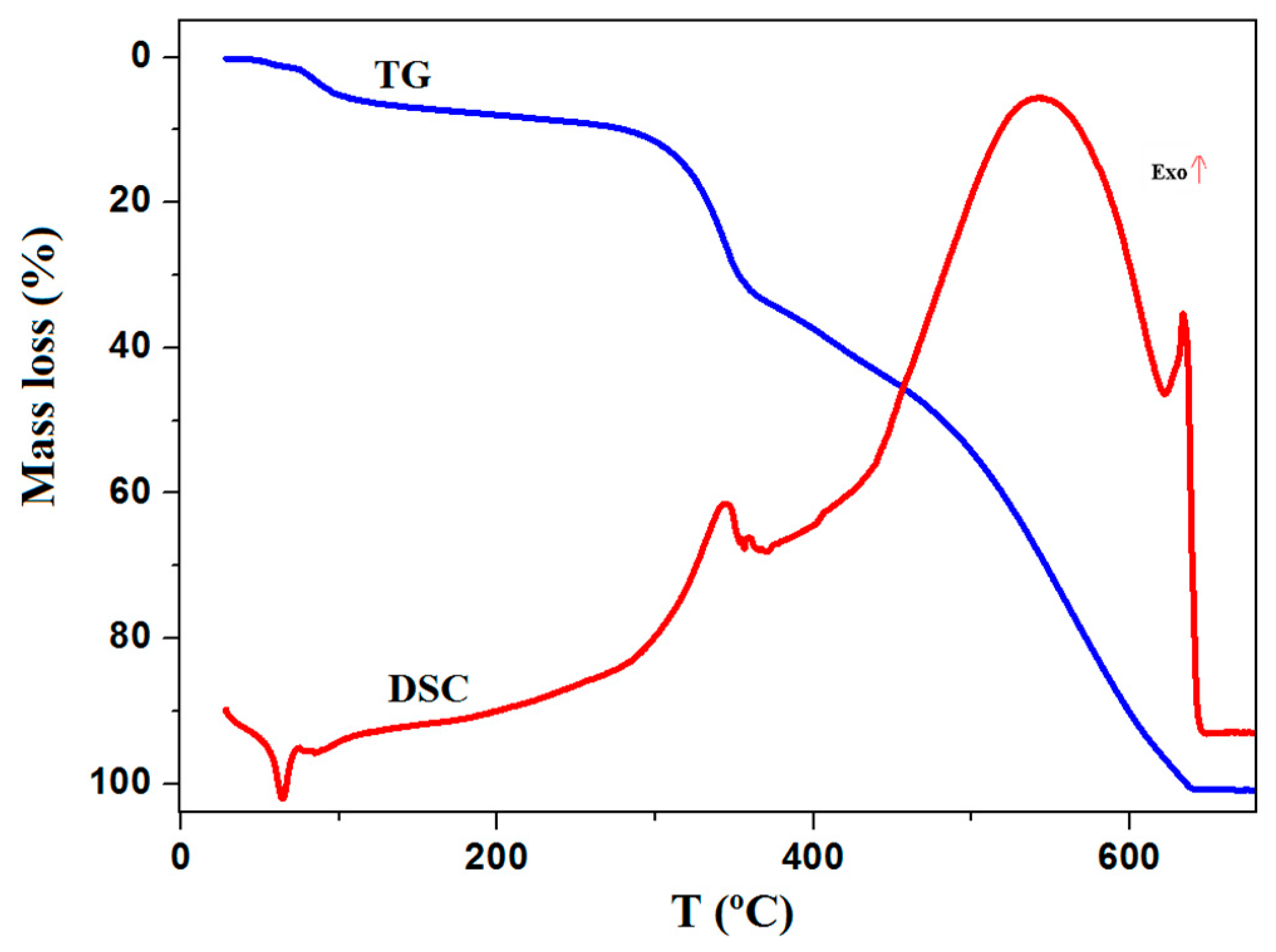
2.2. Thermal Properties
3. Materials and Methods
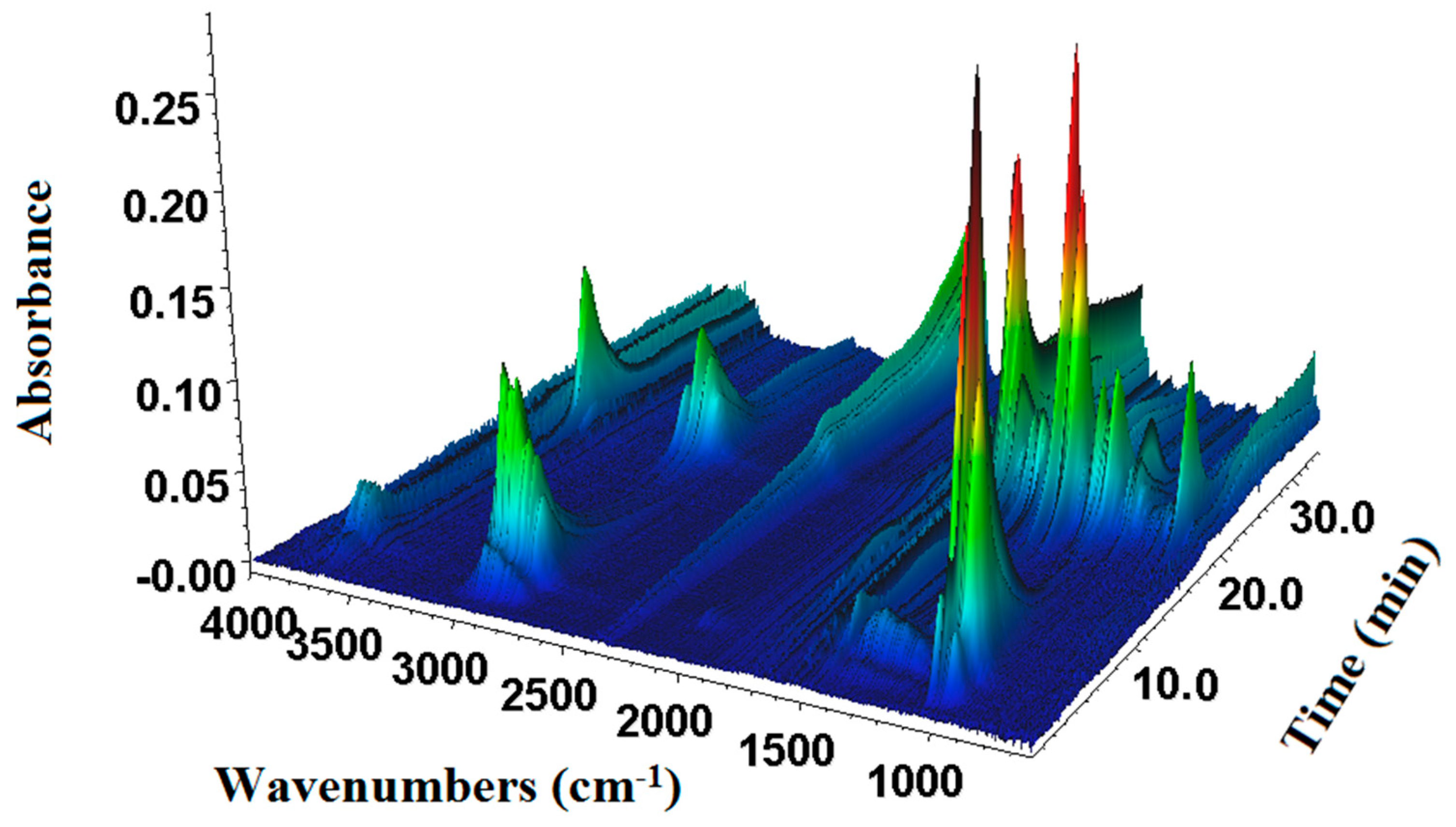
3.1. Synthesis
3.2. X-ray Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Douh, M.H.; Hamid, S.A.; Osman, H.; Ng, S.; Fun, H. 6,6′-Dimethoxy-2,2′-[m-phenylenebis(nitrilomethylidyne)]diphenol. Acta Cryst. 2007, E63, o3570–o3571. [Google Scholar] [CrossRef]
- Ha, K. Crystal structure of N,N′-bis(3-ethoxy-2-hydroxybenzylidene)-phenylene-1,3-diamine, C24H24N2O4. Z. Für Krist. New Cryst. Struct. 2011, 226, 223–224. [Google Scholar] [CrossRef]
- Kargar, H.; Kia, R.; Jamshidvanda, A.; Fun, H. 6,6′-Diethoxy-2,2′-[4,5-dimethyl-ophenylenebis(nitrilomethylidyne)]-diphenol–ethanol–water. Acta Cryst. 2009, E65, o776–o777. [Google Scholar] [CrossRef]
- Akine, S.; Utsuno, F.; Piao, S.; Orita, H.; Tsuzuki, S.; Nabeshima, T. Synthesis, ion recognition ability, and metal-assisted aggregation behavior of dinuclear metallohosts having a bis(saloph) macrocyclic ligand. Inorg. Chem. 2016, 55, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.; Goto, M.; Shimizu, T.; Lee, S.S.; Asakawa, M. Synthesis and characterisation of macrocyclic palladium(II)–sodium(I) complexes: Generation of an unusual metal-mediated electron delocalization. Dalton Trans. 2004, 10, 1513–1515. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Utsuno, F.; Nabeshima, T. Highly efficient regulation of cation recognition and promotion of self-assembly by metalation of a macrocyclic bis(N2O2) ligand with nickel(II). Chem. Commun. 2010, 46, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- van Veggel, F.; Bos, M.; Harkema, S.; van de Bovenkamp, H.; Verboom, W.; Reedijk, J.; Reinhoudt, D. Macrocyclic trinucleating ligands for the cocomplexation of two “soft” (Cu2+, Ni2+, or Zn2+) metal centers and one “hard” (Ba2+ or Cs+) metal center. J. Org. Chem. 1991, 56, 225–235. [Google Scholar] [CrossRef]
- Lakshmipathi, M.; Tothadi, S.; Emmerling, F.; Bhattacharya, B.; Ghosh, S. Different mechanical responses of dimorphic forms of Anthracene Schiffbase crystal. J. Mol. Struct. 2022, 1252, 132182. [Google Scholar] [CrossRef]
- Cuenú-Cabezas, F.; Abonia, R.; Gómez Castaño, J.A. Crystalline derivatives of dipyrazolo-1,5-diazocine and dipyrazolopyrimidine: A case of unexpected synthesis and isostructural polymorphism. Crystals 2022, 12, 714. [Google Scholar] [CrossRef]
- Moulton, B.; Zaworotko, M.J. From molecules to crystal engineering: Supramolecular isomerism and polymorphism in network solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef] [PubMed]
- Sarma, J.A.R.P.; Desiraju, G.R. Polymorphism and pseudopolymorphism in organic crystals—A Cambridge Structural Database study. In Crystal Engineering: The Design and Application of Functional Solids; United States Environmental Protection Agency: Washington, DC, USA, 1999; Volume 539, pp. 325–356. [Google Scholar]
- Omnic Software; Version 2.0.366; Termo Fisher Scientific, Inc.: Medison, WI, USA, 2009.
- CrysAlis PRO; Version 1.171.37.35g; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, UK, 2014.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-Crystal Structure Validation with the Program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osypiuk, D.; Bartyzel, A.; Cristóvão, B. N,N′-Bis(3-ethoxy-2-hydroxybenzylidene)-phenylene-1,3-diamine Methanol Solvate. Molbank 2023, 2023, M1688. https://doi.org/10.3390/M1688
Osypiuk D, Bartyzel A, Cristóvão B. N,N′-Bis(3-ethoxy-2-hydroxybenzylidene)-phenylene-1,3-diamine Methanol Solvate. Molbank. 2023; 2023(3):M1688. https://doi.org/10.3390/M1688
Chicago/Turabian StyleOsypiuk, Dariusz, Agata Bartyzel, and Beata Cristóvão. 2023. "N,N′-Bis(3-ethoxy-2-hydroxybenzylidene)-phenylene-1,3-diamine Methanol Solvate" Molbank 2023, no. 3: M1688. https://doi.org/10.3390/M1688
APA StyleOsypiuk, D., Bartyzel, A., & Cristóvão, B. (2023). N,N′-Bis(3-ethoxy-2-hydroxybenzylidene)-phenylene-1,3-diamine Methanol Solvate. Molbank, 2023(3), M1688. https://doi.org/10.3390/M1688







