Potassium Trifluorotris(pentafluoroethyl)phosphate
Abstract
1. Introduction
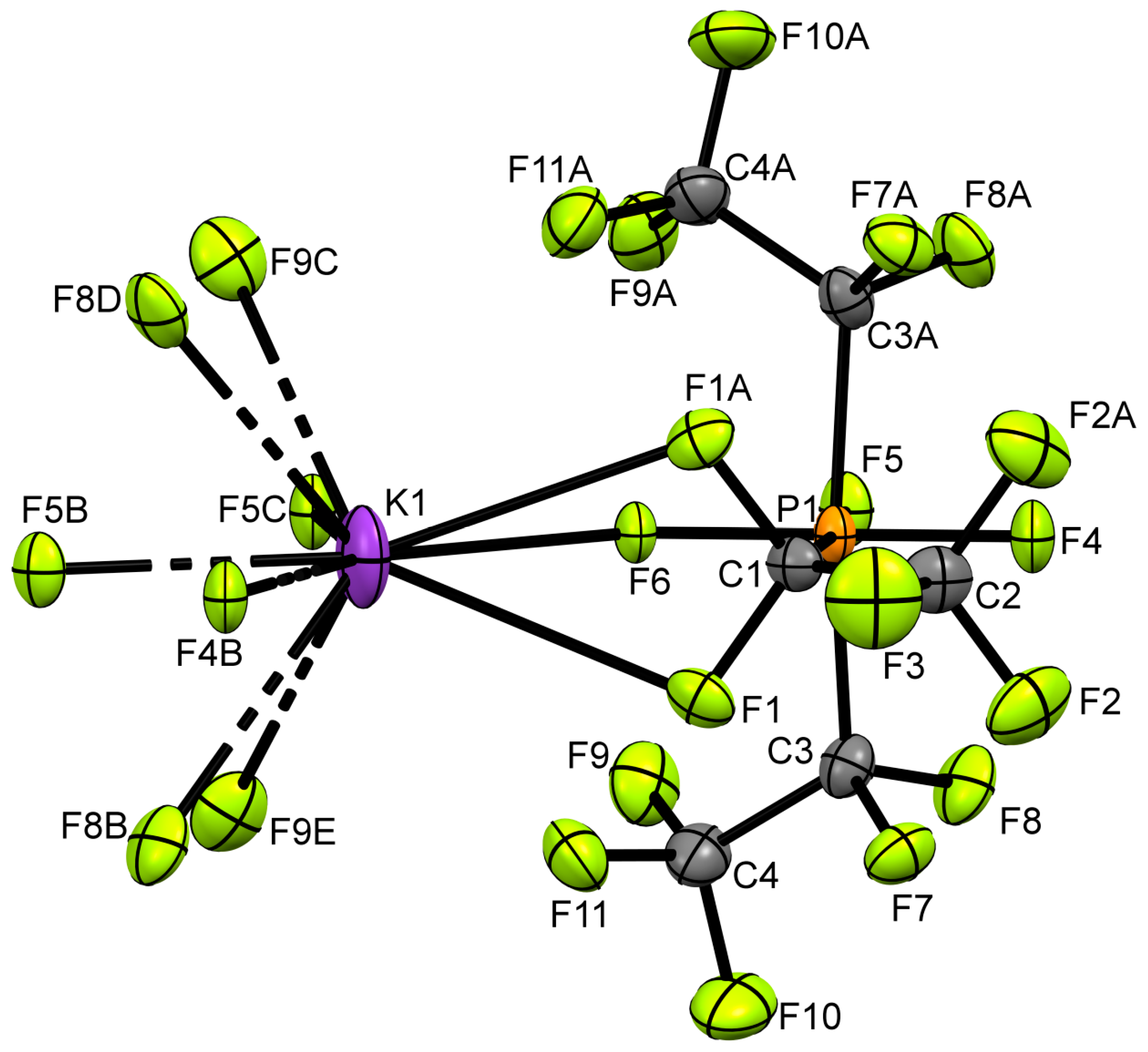
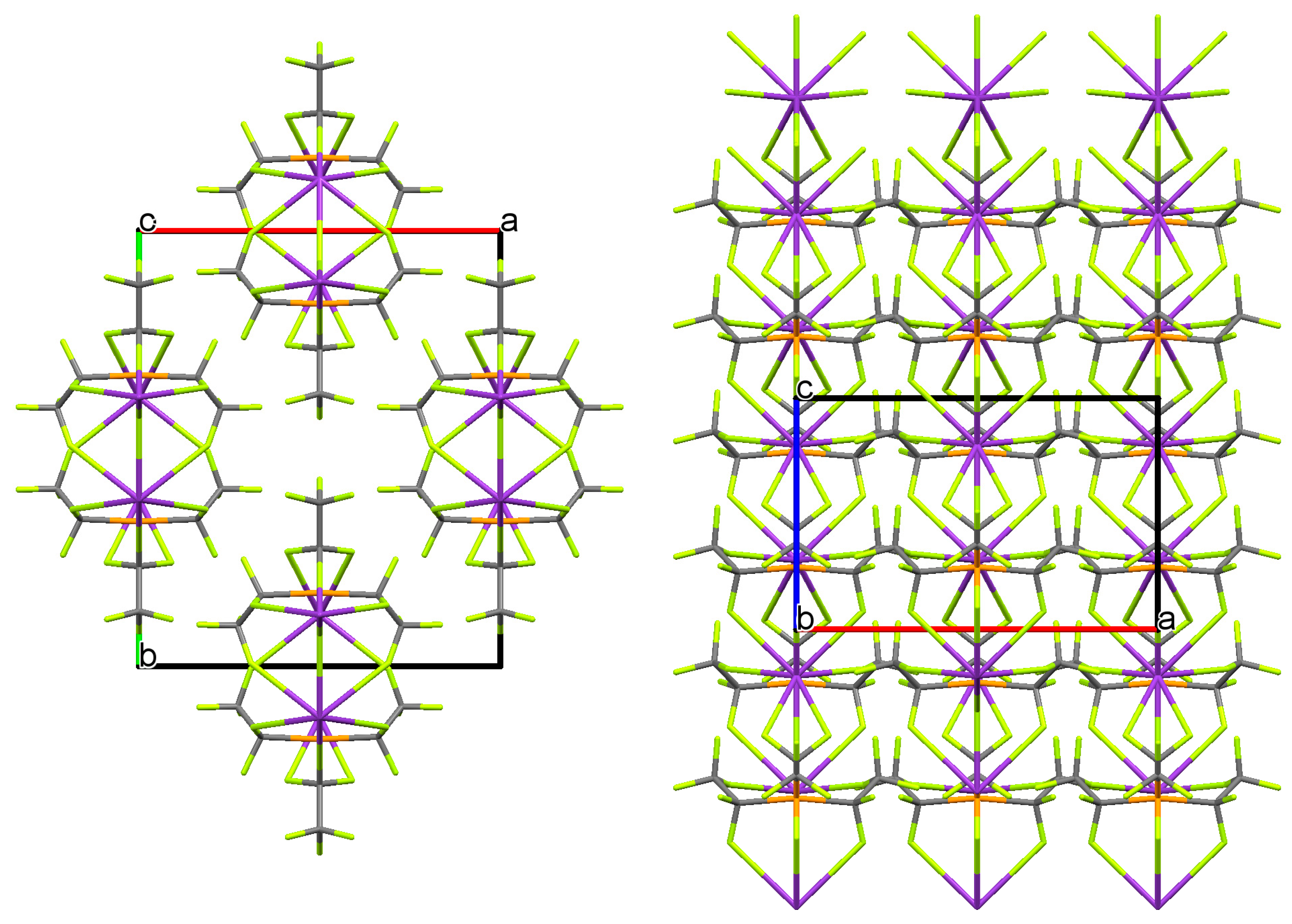
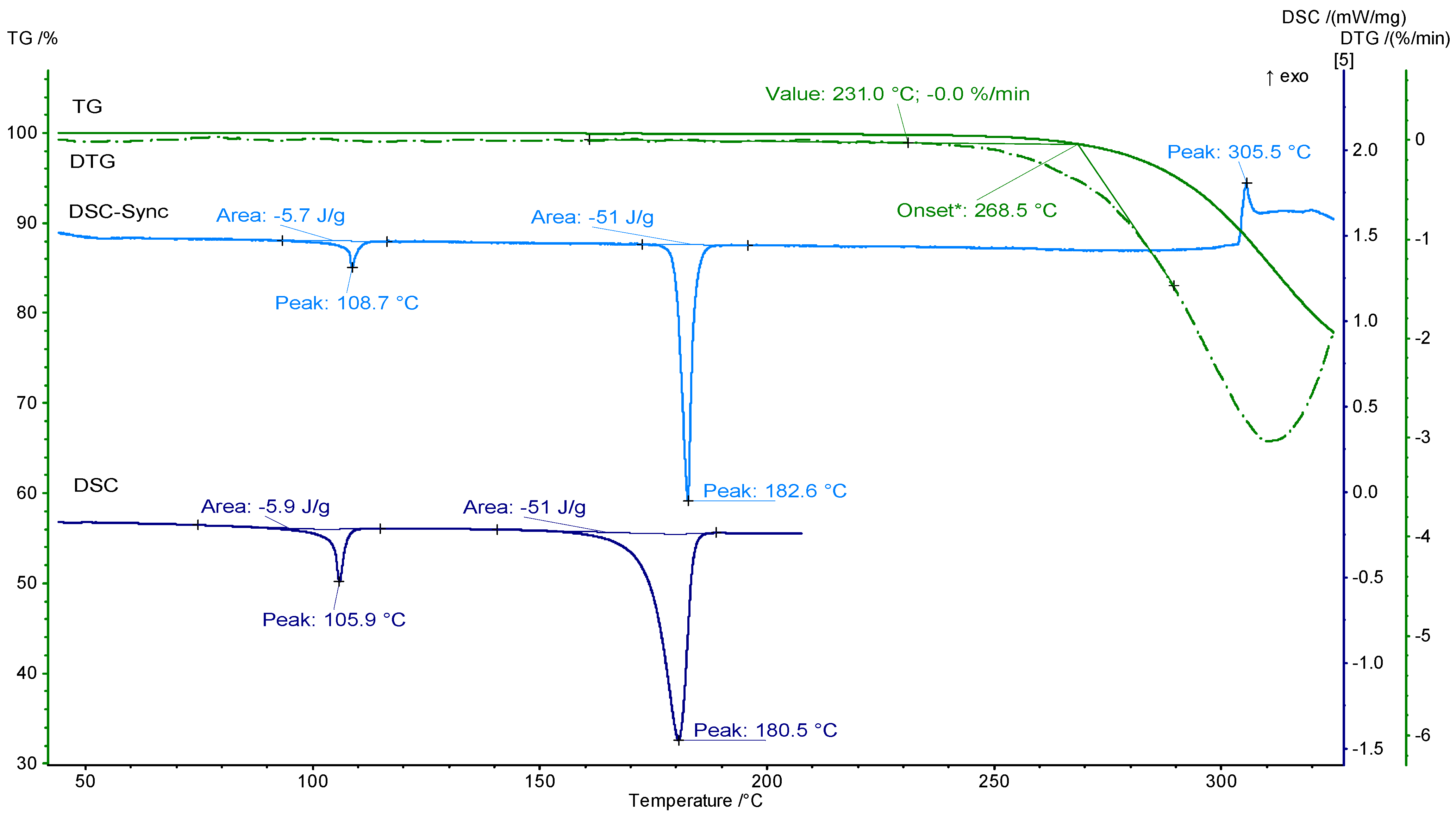
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.3. NMR Data
3.4. Crystal Data of KFAP
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ignat’ev, N.; Sartori, P. Electrochemical fluorination of trialkylphosphines. J. Fluor. Chem. 2000, 103, 57–61. [Google Scholar] [CrossRef]
- Bittner, B.; Koppe, K.; Bilir, V.; Frank, W.; Willner, H.; Ignat’ev, N. Difluorotris(pentafluoroethyl)phosphorene—A highly active catalyst for Diels–Alder reaction. J. Fluor. Chem. 2015, 169, 50–60. [Google Scholar] [CrossRef]
- Bittner, B.; Koppe, K.; Frank, W.; Ignat’ev, N. Michael addition catalyzed by difluorotris (pentafluoroethyl) phosphorane. J. Fluor. Chem. 2016, 182, 22–27. [Google Scholar] [CrossRef]
- Ignat’ev, N.V.; Bader, J.; Koppe, K.; Hoge, B.; Willner, H. Recent progress in perfluoroalkyl-phosphorus chemistry. J. Fluor. Chem. 2015, 171, 36–45. [Google Scholar] [CrossRef]
- Ignat’ev, N.V.; Welz-Biermann, U.; Kucheryna, A.; Bissky, G.; Willner, H. New ionic liquids with tris (perfluoroalkyl) trifluorophosphate (FAP) anions. J. Fluor. Chem. 2005, 126, 1150–1159. [Google Scholar] [CrossRef]
- Ho, T.D.; Zhang, C.; Hantao, L.W.; Anderson, J.L. Ionic liquids in analytical chemistry: Fundamentals, advances, and perspectives. Anal. Chem. 2014, 86, 262–285. [Google Scholar] [CrossRef] [PubMed]
- Minami, I.; Kita, M.; Kubo, T.; Nanao, H.; Mori, S. The tribological properties of ionic liquids composed of trifluorotris (pentafluoroethyl) phosphate as a hydrophobic anion. Tribol Lett. 2008, 30, 215–223. [Google Scholar] [CrossRef]
- Viesca, J.L.; García, A.; Battez, A.H.; González, R.; Monge, R.; Fernández-González, A.; Hadfield, M. FAP—Anion ionic liquids used in the lubrication of a steel–steel contact. Tribol Lett. 2013, 52, 431–437. [Google Scholar] [CrossRef]
- Schmidt, C.; Glück, T.; Schmidt-Naake, G. Modification of Nafion membranes by impregnation with ionic liquids. Chem. Eng. Technol. 2008, 31, 13–22. [Google Scholar] [CrossRef]
- Ignat’ev, N.V.; Finze, M.; Sprenger, J.A.P.; Kerpen, C.; Bernhardt, E.; Willner, H. New hydrophobic ionic liquids with perfluoroalkyl phosphate and cyanofluoroborate anions. J. Fluor. Chem. 2015, 177, 46–54. [Google Scholar] [CrossRef]
- Pavlenko, N.V.; Yagupolsky, L.M. Interaction of tris-(perfluoroalkyl-)phosphine oxides and tris(perfluoroalkyl)difluorophosphoranes with fluoride ion. J. Gen. Chem. 1989, 59, 469–473. [Google Scholar]
- Breitenstein, C.; Ignat’ev, N.; Frank, W. Convenient synthesis of aliphatic (CF3) 2N-compounds. J. Fluor. Chem. 2018, 210, 166–177. [Google Scholar] [CrossRef]
- Pliquett, D.; Schulz, P.S.; Heinemann, F.W.; Bause, A.; Wasserscheid, P. Liquid silver tris (perfluoroethyl) trifluorophosphate salts as new media for propene/propane separation. Phys. Chem. Chem. Phys. 2016, 18, 28242–28253. [Google Scholar] [CrossRef] [PubMed]
- Laus, G.; Schwaerzler, A.; Bentivoglio, G.; Hummel, M.; Kahlenberg, V.; Wurst, K.; Schottenberger, H. Synthesis and crystal structures of 1-alkoxy-3-alkylimidazolium salts including ionic liquids, 1-alkylimidazole 3-oxides and 1-alkylimidazole perhydrates. Z. Naturforsch. B Chem. Sci. 2008, 63, 447–464. [Google Scholar] [CrossRef]
- Solyntjes, S.; Neumann, B.; Stammler, H.; Ignat’ev, N.; Hoge, B. Difluorotriorganylphosphoranes for the Synthesis of Fluorophosphonium and Bismuthonium Salts. Eur. J. Inorg. Chem. 2016, 2016, 3999–4010. [Google Scholar] [CrossRef]
- Weyhing-Zerrer, N.; Kalb, R.; Oßmer, R.; Rossmanith, P.; Mester, P. Evidence of a reverse side-chain effect of tris(pentafluoroethyl)trifluorophosphate [FAP]-based ionic liquids against pathogenic bacteria. Ecotoxicology and environmental safety. Ecotoxicol. Environ. Saf. 2017, 148, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Aoyagi, I.; Takiyama, M.; Matsumoto, K.; Hagiwara, R. Inhibition of Aluminum Corrosion with the Addition of the Tris (pentafluoroethyl) trifluorophosphate Anion to a Sulfonylamide-Based Ionic Liquid for Sodium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 080522. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.37.33; Agilent Technologies: Santa Clara, CA, USA, 2014.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
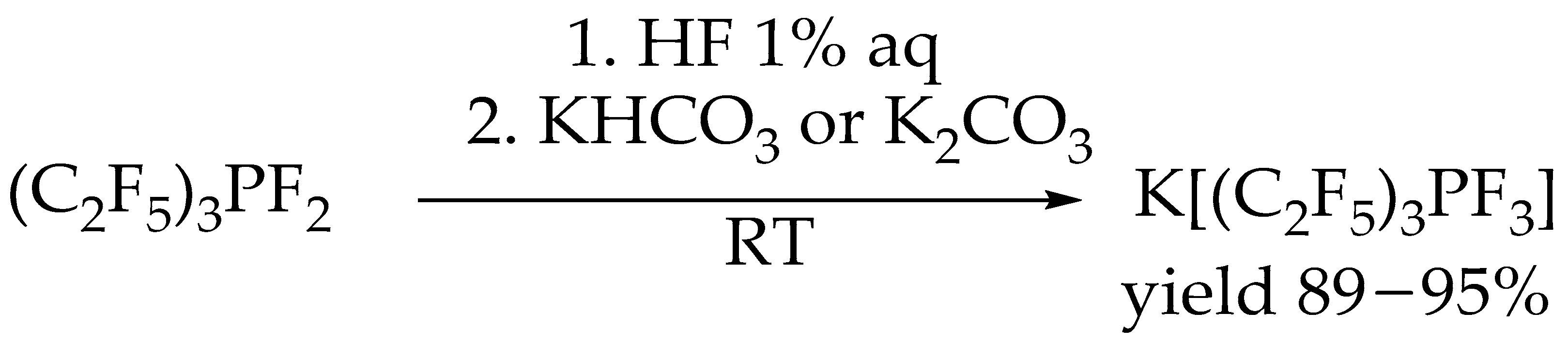
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokrushin, I.G.; Permyakov, P.A.; Pinegina, O.A.; Poturaev, P.S.; Dmitriev, M.V.; Markin, I.V. Potassium Trifluorotris(pentafluoroethyl)phosphate. Molbank 2023, 2023, M1687. https://doi.org/10.3390/M1687
Mokrushin IG, Permyakov PA, Pinegina OA, Poturaev PS, Dmitriev MV, Markin IV. Potassium Trifluorotris(pentafluoroethyl)phosphate. Molbank. 2023; 2023(3):M1687. https://doi.org/10.3390/M1687
Chicago/Turabian StyleMokrushin, Ivan G., Pavel A. Permyakov, Olga A. Pinegina, Petr S. Poturaev, Maksim V. Dmitriev, and Igor V. Markin. 2023. "Potassium Trifluorotris(pentafluoroethyl)phosphate" Molbank 2023, no. 3: M1687. https://doi.org/10.3390/M1687
APA StyleMokrushin, I. G., Permyakov, P. A., Pinegina, O. A., Poturaev, P. S., Dmitriev, M. V., & Markin, I. V. (2023). Potassium Trifluorotris(pentafluoroethyl)phosphate. Molbank, 2023(3), M1687. https://doi.org/10.3390/M1687





