Abstract
Electron-withdrawing heterocyclic units are found in most organic optoelectronic materials. Benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) is an interesting new heterocyclic system, the chemical properties of which are much less studied than other fused thiadiazoles. Cyano derivatives of electron-accepting heterocycles are known as potential components of photoluminescent materials. In this communication, benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole)-4-carbonitrile was successfully obtained via the cyanation of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) with copper(I) cyanide in DMF. The structure of the newly synthesized compound was established by means of elemental analysis, high-resolution mass spectrometry, 1H and 13C NMR, and IR spectroscopy.
1. Introduction
Electron-accepting heterocycles play an important role in the design of organic chromophores due to their ability to reduce the band gap by promoting intramolecular charge transfer [1]. Strong electron-withdrawing building blocks containing thiadiazole rings containing low LUMO energy have attracted much attention [2,3,4,5] because they can be used in the development of various optoelectronic devices such as dye-sensitized solar cells, organic light-emitting diodes and organic field-effect transistors [6]. In recent years, we found that benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) possesses promising electron-accepting properties; its unsubstituted and bromo derivatives successfully participate in aromatic nucleophilic substitution, palladium-catalyzed cross-coupling, and direct C-H arylation [7,8]. The selective synthesis of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 1 [9] and the successful selective substitution of bromine or hydrogen atoms [10] made it possible to lay the foundation for the efficient synthesis of unsymmetrical derivatives of this heterocyclic system. Cyano derivatives of electron-accepting heterocycles, namely 2,1,3-benzothiadiazole, are shown to have interesting physical properties for the design of push–pull dyes [11], thermally activated delayed fluorescence sensitizers [12], mediators for electrocatalytic hydrogen evaluation on glassy carbon electrodes [13], and others. In continuation of our study of the reactivity of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 1, we report its cyanation reaction to benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole)-4-carbonitrile 2.
2. Results and Discussion
The cyanation of bromo-benzo-bis-thiadiazoles has not been described in the literature. A search of the literature on the Reaxys and SciFinder databases for the cyanation of 4-bromo-2,1,3-benzothiadiazoles showed that the most common methods are heating at high temperature with copper(I) cyanide in DMF [14,15] or in NMP [16,17,18]. We have shown that bromide 1 reacted with copper (I) cyanide in both solvents under heating (Scheme 1). It turned out that the nature of the solvent, as well as the reaction temperature, significantly affected the course of chemical transformation. The use of NMP as a solvent in the reaction with copper(I) cyanide led to the complete decomposition of the starting bromide 1 (Table 1, entries 1,2). The treatment of bromide 1 with CuCN in DMF at a temperature of 80 °C gave the cyanation product 2 in trace amounts due to its very slow formation (Table 1, entry 3). An increase in the reaction temperature led to an increase in the yield of the target product 2; the highest yield was achieved at 140 °C (Table 1, entry 5). Other attempts to improve the yield of cyanide 2 were unsuccessful; a reaction with potassium cyanide in DMF or with zinc(II) cyanide in the presence of a tetrakis(triphenylphosphine)palladium catalyst (Pd(PPh3)4) in NMP [19] only led to a slow decomposition of the starting bromide 1 (Table 1, entries 6,7).
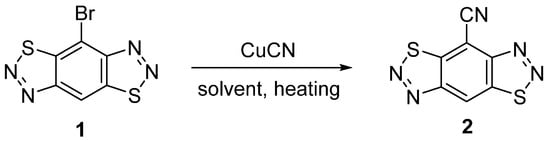
Scheme 1.
Synthesis of benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole)-4-carbonitrile 2.

Table 1.
Cyanation of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 1.
The structure of benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole)-4-carbonitrile 2 was confirmed by means of elemental analysis, high-resolution mass spectrometry, 1H, 13C NMR, and IR spectroscopy. The presence of a cyano group in compound 2 was evidenced by the appearance of a band at 2233 cm–1 in the IR spectrum and an intense signal in the 13C; NMR spectrum (δ = 119.3 ppm).
In conclusion, benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole)-4-carbonitrile 2 was synthesized via the cyanation of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 1 with copper(I) cyanide in DMF. The compound obtained may serve as a precursor for the preparation of unsymmetrical 4,7-disubstituted benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazoles) containing a cyano group as organic optoelectronic materials via palladium-catalyzed C–H direct arylation reactions with aryl and thienyl halogenides [10].
3. Materials and Methods
4-Bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 1 was prepared according to the published method [9]. The solvents and reagents were purchased from commercial sources and used as received. Elemental analysis was performed on a 2400 Elemental Analyzer (Perkin Elmer Inc., Waltham, MA, USA). The 1H and 13C NMR spectra were determined with a Bruker AM-300 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA) (at frequencies of 300 and 75 MHz) in CDCl3 solution. The high-resolution MS spectrum was measured on a Bruker micrOTOF II instrument (Bruker Daltonik GmbH, Bremen, Germany) using electrospray ionization (ESI). The IR spectrum was measured with a Bruker “Alpha-T” instrument (Bruker Corporation, Billerica, Massachusetts, USA) in a KBr pellet.
CuCN (161 mg, 1.81 mmol) was added to a solution of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 1 (500 mg, 1.81 mmol) in anhydrous DMF (20 mL). The resulting mixture was degassed with argon in a sealed vial and then stirred at 140 °C for 24 h. On completion (monitored using TLC), water (80 mL) was added to the reaction mixture, and the organic layer was extracted with CH2Cl2 (3 × 70 mL), dried with MgSO4 and then concentrated in vacuo. The residue was purified via column chromatography on silica gel (Silica gel Merck 60, eluent hexane–CH2Cl2, 1:1, v/v). Yield 257 mg (65%), red solid, Rf = 0.3 (hexane–CH2Cl2, 1:1, v/v). Mp = 186–187 °C. IR spectrum, ν, cm–1: 3077 and 2923 (CH), 2233 (CN), 1337, 1289, 1235, 1212, 880, 846, 815, 678, 551, 523. 1H NMR (ppm): δ 9.59 (s, 1H, CH). 13C NMR (ppm): δ 157.7, 157.1, 143.8, 140.3, 119.3 (CN), 113.8, 98.7. HRMS (ESI-TOF), m/z: calcd for C7HN5S2Ag [M+Ag]+, 325.8719, found, 325.8726. Anal. calcd. for C7HN5S2 (219.25): C, 38.35; H, 0.46; N, 31.94. Found: C, 38.20; H, 0.43; N, 31.82%.
Supplementary Materials
The following are available online: copies of 1H, 13C NMR, IR, and HR mass spectra for compound 2.
Author Contributions
Conceptualization, O.A.R.; methodology, T.N.C.; software, T.N.C.; validation, O.A.R.; formal analysis, investigation, T.N.C., T.A.K. and K.S.G.; resources, T.N.C.; data curation, T.N.C.; writing—original draft preparation, O.A.R.; writing—review and editing, O.A.R.; visualization, O.A.R.; supervision, O.A.R.; project administration, O.A.R.; funding acquisition, O.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of compound 2 are available from the authors.
References
- Takimiya, K.; Osaka, I.; Nakano, M. π-Building Blocks for Organic Electronics: Revaluation of “Inductive” and “Resonance” Effects of π-Electron Deficient Units. Chem. Mater. 2014, 26, 587–593. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Rakitin, O.A. Benzobischalcogenadiazoles: Synthesis and applications (microreview). Chem. Heterocycl. Compd. 2022, 58, 307–309. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Mikhalchenko, L.V.; Golovanov, I.S.; Amelichev, S.A.; Rakitin, O.A. Synthesis of the 4,7-Dibromo Derivative of Highly Electron-Deficient [1,2,5]Thiadiazolo[3,4-d]pyridazine and Its Cross-Coupling Reactions. Eur. J. Org. Chem. 2018, 2018, 5668–5677. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ono, K.; Tomura, M.; Tanaka, S. Synthesis and Properties of Benzobis(thiadiazole)s with Nonclassical π-Electron Ring Systems. Tetrahedron 1997, 53, 10169–10178. [Google Scholar] [CrossRef]
- Bianchi, L.; Zhang, X.; Chen, Z.; Chen, P.; Zhou, X.; Tang, Y.; Liu, B.; Guo, X.; Facchetti, A. New Benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (iso-BBT)-Based Polymers for Application in Transistors and Solar Cells. Chem. Mater. 2019, 31, 6519–6529. [Google Scholar] [CrossRef]
- Iftikhar, R.; Khan, F.Z.; Naeem, N. Recent synthetic strategies of small heterocyclic organic molecules with optoelectronic applications: A review. Mol. Divers. 2023, 27, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Chmovzh, T.N.; Alekhina, D.A.; Kudryashev, T.A.; Rakitin, O.A. Efficient Synthesis of 4,8-Dibromo Derivative of Strong Electron-Deficient Benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) and Its SNAr and Cross-Coupling Reactions. Molecules 2022, 27, 7372. [Google Scholar] [CrossRef] [PubMed]
- Chmovzh, T.N.; Kudryashev, T.A.; Alekhina, D.A.; Rakitin, O.A. Palladium-Catalyzed Direct (Het)arylation Reactions of Benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole and 4,8-Dibromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole). Molecules 2023, 28, 3977. [Google Scholar] [CrossRef] [PubMed]
- Chmovzh, T.N.; Alekhina, D.A.; Kudryashev, T.A.; Rakitin, O.A. 4-Bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole). Molbank 2022, 2022, M1362. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Alekhina, D.A.; Kudryashev, T.A.; Aysin, R.R.; Korlyukov, A.A.; Rakitin, O.A. Benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) and Its Bromo Derivatives: Molecular Structure and Reactivity. Int. J. Mol. Sci. 2023, 24, 8835. [Google Scholar] [CrossRef] [PubMed]
- Thooft, A.M.; Cassaidy, K.; VanVeller, B. A Small Push–Pull Fluorophore for Turn-on Fluorescence. J. Org. Chem. 2017, 82, 8842–8847. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-K.; Huang, C.-C.; Kumar, S.; Li, S.-H.; Dong, Z.-L.; Fung, M.-K.; Jiang, Z.-Q.; Liao, L.-S. Thermally activated delayed fluorescence sensitizer for D–A–A type emitters with orange-red light emission. J. Mater. Chem. C 2018, 6, 10030–10035. [Google Scholar] [CrossRef]
- Axelsson, M.; Marchiori, C.F.N.; Huang, P.; Araujo, C.M.; Tian, H. Small Organic Molecule Based on Benzothiadiazole for Electrocatalytic Hydrogen Production. J. Am. Chem. Soc. 2021, 143, 21229–21233. [Google Scholar] [CrossRef] [PubMed]
- Odella, E.; Secor, M.; Elliott, M.; Groy, T.L.; Moore, T.A.; Hammes-Schiffer, S.; Moore, A.L. Multi PCET in symmetrically substituted benzimidazoles. Chem. Sci. 2021, 12, 12667–12675. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Tsai, C.-H.; Lin, F.; Huang, T.-W.; Chou, S.-H.; Wu, C.-C.; Wong, K.-T. 2,1,3-Benzothiadiazole-containing donor–acceptor–acceptor dyes for dye-sensitized solar cells. Tetrahedron 2012, 68, 7509–7516. [Google Scholar] [CrossRef]
- Gampe, D.M.; Nöller, F.; Hänsch, V.G.; Schramm, S.; Darsen, A.; Habenicht, S.H.; Ehrhardt, S.; Weiß, D.; Görls, H.; Beckert, R. Surprising characteristics of D–A-type functional dyes by introducing 4-alkoxythiazoles as the donor-unit. Tetrahedron 2016, 72, 3232–3239. [Google Scholar] [CrossRef]
- Yu, Y.; Xing, H.; Zhou, Z.; Liu, J.; Sung, H.H.-Y.; Williams, I.D.; Halpert, J.E.; Zhao, Z.; Tang, B.Z. How do molecular interactions affect fluorescence behavior of AIEgens in solution and aggregate states? Sci. China Chem. 2022, 65, 135–144. [Google Scholar] [CrossRef]
- Yu, Y.; Xing, H.; Liu, D.; Zhao, M.; Sung, H.H.-Y.; Williams, I.D.; Lam, J.W.Y.; Xie, G.; Zhao, Z.; Tang, B.Z. Solution-processed AIEgen NIR OLEDs with EQE Approaching 15%. Angew. Chem. Int. Ed. 2022, 61, e202204279. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Kudryashev, T.A.; Rakitin, O.A. (E)-7-(4-(Diphenylamino)styryl)benzo[c][1,2,5]thiadiazole-4-carbonitrile. Molbank 2022, 2022, M1385. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).