Abstract
Herein, we report the synthesis of 2-(2-fluoro-[1,1′-biphenyl]-4-yl)-N-(4-methyl-2-oxo-2H-chromen-7-yl)propanamide in the reaction between 7-amino-4-methyl-2H-chromen-2-one and (±)-flurbiprofen. The newly-obtained bio-functional hybrid compound was fully characterized via 1H, 13C NMR, UV, and mass spectral data.
1. Introduction
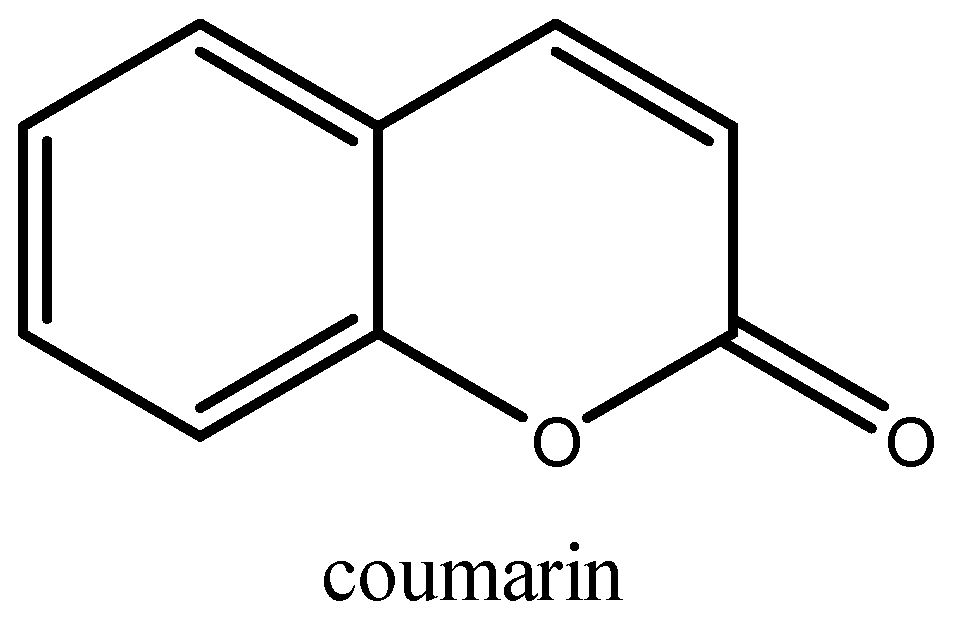
Isolated first in 1820 from Tonka beans [1], coumarin and its derivatives still draw much attention from organic chemists. Coumarin, with the chemical formula C9H6O2, is a heterocyclic molecule generated by the fusion of a pyrone ring with benzene (Figure 1). Thousands of natural coumarins isolated, structurally characterized, synthesized, and biologically active from plants, bacteria, fungi, and chemical synthesis have been described since then [2].
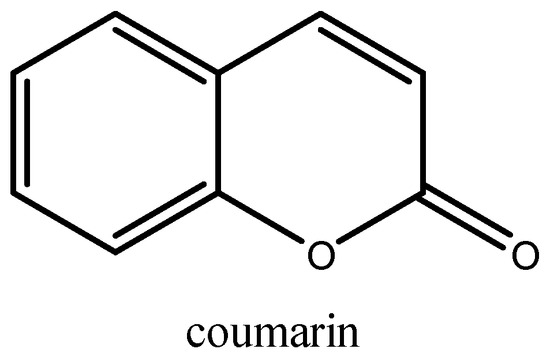
Figure 1.
Structural formula of coumarin.
Coumarin has received a lot of attention because its skeleton is found in many biologically active drugs. Coumarin and its derivatives are thought to be the most active classes of heterocycles, with a wide range of biological action. They have been shown to be effective as antibacterial [3], antifungal [4], anti-inflammatory [5], antidepressant [6], anti-HIV [7], and antitumor medicines [8].
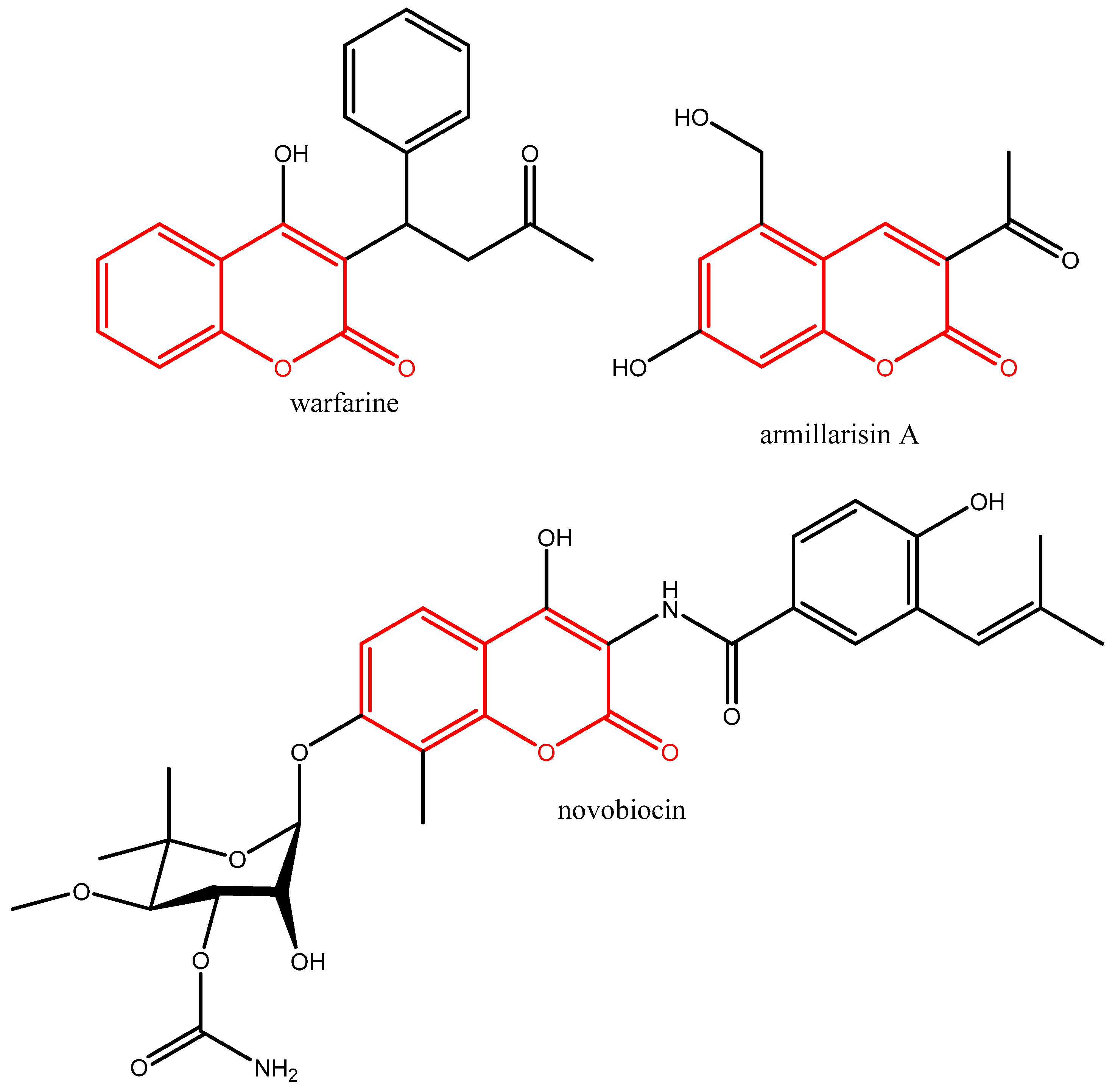
Notable examples of commercialized medications based on the coumarin scaffold (given in red colour in Figure 2) include the anticoagulant warfarin, the choleretic armillarisin A, and the antibacterial novobiocin (Figure 2). Warfarin is an anticoagulant that inhibits coagulation rather than decreasing viscosity. It is commonly used to prevent blood clots in the circulatory system such as deep vein thrombosis and pulmonary embolism. Armillarisin A is an antibiotic [9] and novobiocin is an aminocoumarin antibiotic that is produced by the actinomycete Streptomyces niveus (Figure 2).
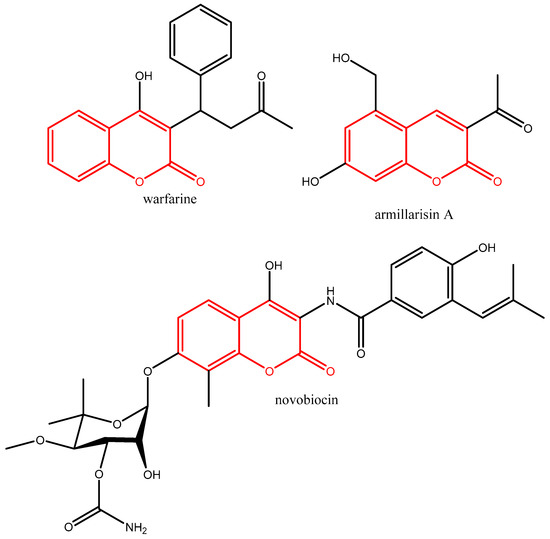
Figure 2.
Structural formulas of warfarin, armillarisin A, and novobiocin.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used to treat pain and inflammation all over the world [10]. A very popular group of NSAIDs is the profen group. Profens are a class of nonselective, nonsteroidal anti-inflammatory medications that lower pain, body temperature in fever, symptoms of inflammation, and the development of cancer in mice [11]. Profens have a strong affinity for plasma proteins. Each profen has a chiral center, leading to the synthesis of two enantiomers (R and S). The profens are generally available as racemates, which are equal mixes of the R and S stereoisomers.
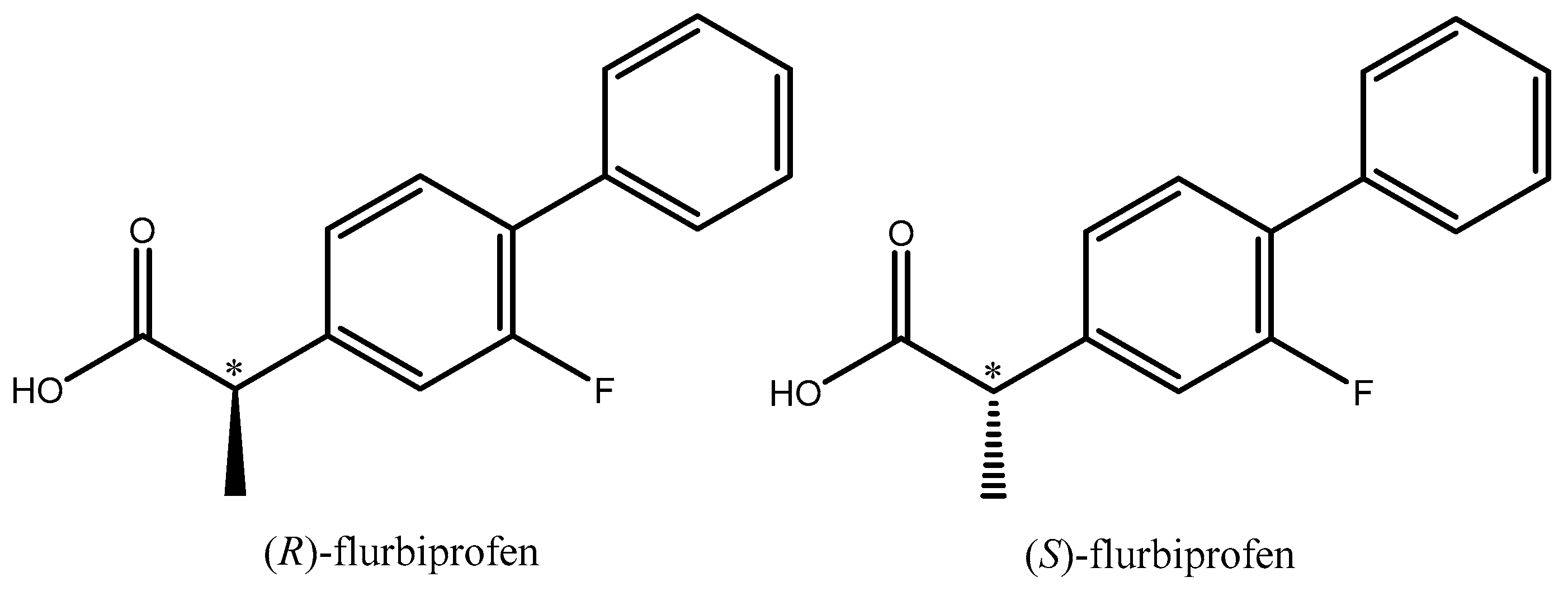
Flurbiprofen, also known as 2-(2-fluoro-[1,1′-biphenyl]-4-yl) propionic acid, is a well-known analgesic, antipyretic, and anti-inflammatory NSAID. Flurbiprofen is a 2-arylpropionic acid chiral derivative. Although it has a chiral core, the S-(+)-enantiomer is responsible for the majority of the beneficial anti-inflammatory activity, but both enantiomers can be analgesic, and all flurbiprofen formulations sold to date are racemates (Figure 3).
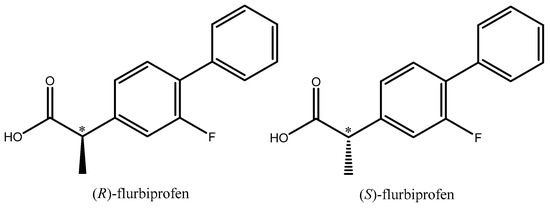
Figure 3.
Structural formula of R- and S-flurbiprofen.
The racemic mixture of flurbiprofen is a potent inhibitor of prostaglandin synthesis. Flurbiprofen has a strong analgesic effect and is thus utilized for the short-term alleviation of postoperative pain in individuals with dental difficulties. Its application as an anti-inflammatory drug in the treatment of eye problems has also been investigated. [12].
The pharmaceutical business relies heavily on amide synthesis. Amides can be made by a variety of chemical processes. Nucleophilic acyl substitution, partial hydrolysis of nitriles, and the coupling of amines with carboxylic acids in the presence of DCC, EDC, or other “dehydrating” chemicals are three of the most efficient procedures for the synthesis of amides.
Obtaining a new hybrid molecule built from a flurbiprofen fragment connected to a coumarin skeleton via an amide bond on position 7 is especially intriguing for studying its bioactivity.
2. Results
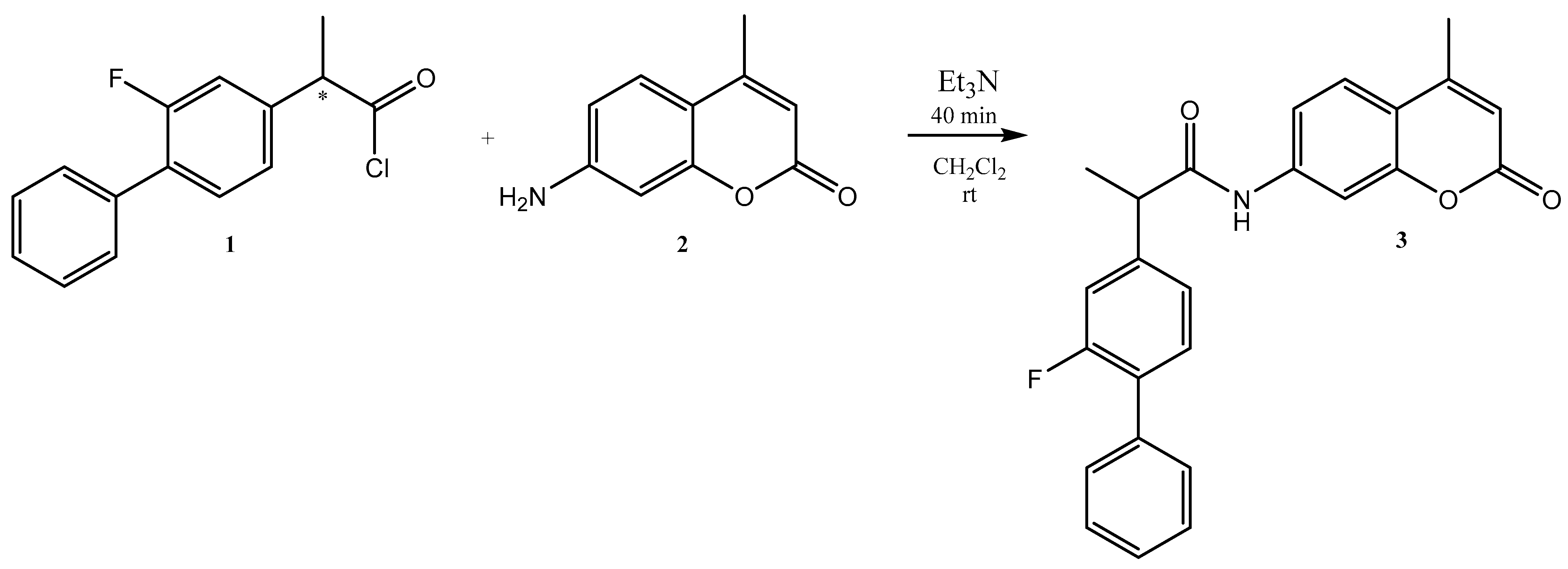
Herein, we report the successful synthesis of 2-(2-fluoro-[1,1′-biphenyl]-4-yl)-N-(4-methyl-2-oxo-2H-chromen-7-yl)propanamide 3, as shown in Scheme 1. For this purpose, 2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanoyl chloride 1 (1 mmol) was added to the solution of 7-amino-4-methyl-2H-chromen-2-one 2 (1 mmol) in dichloromethane. After stirring the reaction mixture for 10 min, an excess of trimethylamine (1.5 mmol) was carefully added. After 30 min, the inspected TLC demonstrated the acquisition of the final product 3. The Schotten–Baumann reaction provides the simple formation of amide functions from amines and acyl chlorides. Because we employed racemic flurbiprofen, we obtained a product that was a mixture of two enantiomers.
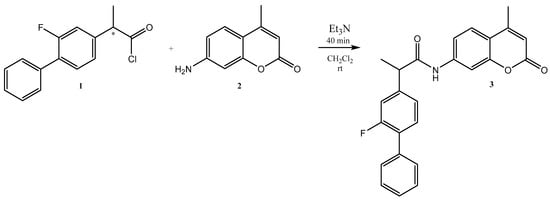
Scheme 1.
Synthesis of 2-(2-fluoro-[1,1′-biphenyl]-4-yl)-N-(4-methyl-2-oxo-2H-chromen-7-yl)propanamide.
When observing the new hybrid’s 1H NMR spectral data, the signals for all 20 protons can be seen very clearly (Figure S1). A doublet for three protons for the CH3 group is seen at 1.50 ppm. A doublet for the methyl group at position 4 in the coumarin skeleton can be seen at 2.38 ppm. At 3.96 ppm, we observe a quartet for the H atom connected to the enantiomeric carbon in the flurbiprofen moiety. The signal for the H (CH) between the double bond and the C=O function appears at 6.25 ppm. The signal for the NH is a singlet at 10.60 ppm. In the area between 7.30 and 7.79 ppm, all 11 aromatic protons are observed.
The 13C NMR spectra confirm the structure of the newly discovered hybrid molecule 3. The two carbonyl carbons C=O are visible at 172.88 ppm and 160.46 ppm, respectively. The more left-shifted (172.88 ppm) is for the carbonyl carbon of the flurbiprofen residue and the one at 160.46 ppm is the carbonyl from the coumarin molecule. A spin–spin interaction with Fluorine 19 is detected immediately after the carbonyl carbons at 159.30 ppm (d, 1JC-F =234.1 Hz), the second doublet at 115.48 ppm (d, 2JC-F = 23.1 Hz), and the third one at 131.24 (d, 3JC-F = 3.7 Hz). The signal for the CH3 group from the profen residue is at 18.41 ppm and the one from the coumarin frame is shown at 18.89 ppm. The chiral carbon appears at 46.15 ppm (Figure S2).
In prior research, we discovered that the fragmentation of profen derivatives is primarily caused by amide bond cleavage. The result is a profen cation that is resonance stable [13]. It is ion m/z 199 (C14H12F+) in this specific instance, which is the flurbiprofen cation (Figure S5). The 7-amino-4-methyl coumarin cation is represented by the ion m/z 176 (C10H10NO2+), which is produced when the N-C link is broken (Figure S5). With the removal of neutral CO and CO2 molecules, the same ion undergoes rearrangement, resulting in the ions m/z 148 (C9H10NO+), m/z 132 (C9H10N+), and m/z 120 (C8H10N+) (Figure S5). The production of the ion m/z 115 (C9H7+) is caused by the separation of the neutral molecule NH3 from the ion m/z 132 (Figure S5).
3. Materials and Methods
All reagents and chemicals were purchased commercially (Sigma-Aldrich S.A. and Riedel-de Haën, Sofia, Bulgaria) and used as received. The NMR spectral data were recorded on a Bruker Avance Neo 400 spectrometer (BAS-IOCCP—Sofia, Bruker, Billerica, MA, USA). 1H NMR and 13C NMR spectra for all compounds were taken in DMSO-d6 at 400 MHz and 101 MHz, respectively. Chemical shifts are given in relative ppm and were referenced to tetramethylsilane (TMS) (δ = 0.00 ppm) as an internal standard; the coupling constants are indicated in Hz. The NMR spectra were recorded at room temperature (ac. 295 K). The melting point was determined on a Boetius hot stage apparatus and is uncorrected. Absorbance was measured with a spectrophotometer Camspec M508, Leeds, UK. The MS analysis was performed on a Q Exactive Plus high-resolution mass spectrometer (HRMS) with a heated electrospray ionization source (HESI-II) (Thermo Fisher Scientific, Inc., Bremen, Germany) equipped with a Dionex Ultimate 3000RSLC ultrahigh-performance liquid chromatography (UHPLC) system (Thermo Fisher Scientific, Inc., Waltham, MA, USA). TLC was carried out on precoated 0.2 mm Fluka silica gel 60 plates (Merck KGaA, Darmstadt, Germany). IR spectra were measured on a Bruker Alpha II FT IR spectrometer.
3.1. (±)-(2-(2-Fluoro-[1,1′-biphenyl]-4-yl)propanoyl chloride 1
For (±)-flurbiprofen 1 (1.0 mmol, 0.244 g) dissolved in toluene (30 mL), an excess of thionyl chloride (1.2 mmol, 0.087 mL) was added. The reaction mixture was stirred under reflux for two hours. The excess of thionyl chloride and the toluene were removed under reduced pressure. The obtained (±)-2-(6-Methoxynaphthalen-2-yl)propanoyl chloride 1 was used without further purification.
3.2. Synthesis of (±)2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N-(4-methyl-2-oxo-2H-chromen-7-yl)propanamide 3
In a solution of 7-amino-4-methyl coumarin 2 (1.0 mmol, 0.175 g) in dichloromethane (30 mL) an equal amount of ((±)-(2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanoyl chloride 3 (1 mmol, 0.401 g) was added. After 10 min, triethylamine (1.2 mmol, 1.67 mL) was added to the solution. After 30 min, the solution was washed with diluted hydrochloric acid, a saturated solution of Na2CO3, and brine. The combined organic layers were dried over anhydrous Na2SO4 and the solvent was removed under reduced pressure. The new hybrid molecule was purified by filtration through short-column chromatography over neutral Al2O3.
3.3. 2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N-(4-methyl-2-oxo-2H-chromen-7-yl)propanamide
White solid (m.p. 162–164 °C), yield 91% (0.364 g), Rf = 0.4 (petroleum/diethyl ether = 1/4), 1H NMR (400 MHz, DMSO) δ 10.60 (s, 1H), 7.79 (d, J = 2.0 Hz, 1H), 7.70 (d, J = 8.7 Hz, 1H), 7.54–7.52 (m, 2H), 7.51–7.48 (m, 2H), 7.47–7.44 (m, 2H), 7.41–7.38 (m, 1H), 7.36–7.30 (m, 2H), 6.25 (d, J = 1.4 Hz, 1H), 3.96 (q, J = 6.9 Hz, 1H), 2.38 (d, J = 1.3 Hz, 3H), 1.50 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 172.88 (C=O), 160.46 (O-C=O), 159.30 (d, 1JC-F =234.1 Hz) 158.14 (C-Ar), 154.10 (C-Ar), 153.49 (C-Ar), 143.73 (C-Ar), 142.85 (C-Ar), 135.33 (C-Ar), 131.24 (d, 3JC-F = 3.7 Hz), 131.25 (C-Ar), 129.16 (C-Ar), 129.06 (C-Ar), 128.26 (C-Ar), 127.31 (C-Ar), 126.37 (C-Ar), 124.32 (C-Ar), 115.71 (C-Ar), 115.56 (C-Ar), 115.48 (d, 2JC-F = 23.1 Hz), 115.37 (C-Ar), 112.80 (C-Ar), 111.63 (C-Ar), 106.17 (C-Ar), 98.98 (C-Ar), 46.15 (CH), 18.89 (C=C-CH3), 18.41 (CHCH3). UV λmax, MeOH: 231 (ε = 20,000) nm, 254 (ε = 16,900) nm, 348 (ε = 12,600) nm. HRMS Electrospray ionization (ESI) m/z calcd for [M+H]+ C25H21FNO3+ = 402.1500, found 402.1495 (mass error ∆m = −1.24 ppm), calcd for [M+Na]+ C25H20FNO3Na+ = 424.1319, found 424.1313 (mass error ∆m = −1.41 ppm).
Supplementary Materials
The following supporting information can be downloaded online. Figure S1: 1H-NMR spectrum of compound 3; Figure S2: 13C-NMR spectrum of compound 3; Figure S3: UV spectrum of compound 3; Figure S4: ESI-HRMS of compound 3; Figure S5: Mass spectrum of 3 obtained by positive ion ESI-MS/MS. Proposed fragmentation of protonated 3.
Author Contributions
Conceptualization, I.I. and S.M.; methodology, S.M.; software, S.M.; validation, D.B., S.M. and I.I.; formal analysis, S.M., D.B. and P.N.; investigation, S.M. and D.B.; resources, I.I.; data curation, S.M.; writing—original draft preparation, S.M. and D.B.; writing—review and editing, S.M., D.B. and I.I.; visualization, S.M.; supervision, I.I.; project administration, S.M.; funding acquisition, I.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project ΦΠ23-XΦ-005.
Data Availability Statement
The data presented in this study are available in this article and supporting Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vogel, A. Darstellung von Benzoesäure aus der Tonka-Bohne und aus den Meliloten-der Steinklee-Blumen. ADP 1820, 64, 161–166. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- El-Saghier, A.; Khodairy, A.; Khodiyar, A. New synthetic approaches to condensed and spiro coumarins: Coumarin-3-thiocarboxamide as building block for the the synthesis of condense and spiro coumarins. Phosphorus Sulfur. 2000, 160, 105–119. [Google Scholar] [CrossRef]
- Satyanarayan, V.; Sreevani, P.; Sivakumar, A. Synthesis and antimicrobial activity of new Schiff bases containing coumarin moiety and their spectral characterization. Arkivoc 2008, 17, 221–233. [Google Scholar] [CrossRef]
- Garazd, M.; Muzychka, O.; Voyk, A.; Nagorichna, I.; Ogorodniichuk, A. Modified coumarins. 27. Synthesis and antioxidant activity of 3-substituted 5,7-dihydroxy-4-methylcoumarins. Chem. Nat. Compd. 2007, 43, 19–23. [Google Scholar] [CrossRef]
- Sashidhara, K.; Modukuri, R.; Singh, S.; Rao, K.; Teja, G.; Gupta, S.; Shukla, S. Design and synthesis of new series of coumarin-aminopyran derivatives possessing potential anti-depressant-like activity. Bioorg. Med. Chem. Lett. 2015, 25, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kumamoto, T.; Ishikawa, T. Enantioselective total synthesis of anti HIV-1 active (+)calanolide A through a quinine-catalyzed asymmetric intramolecular oxo-Michael addition. Tetrahedron Lett. 2000, 41, 10229–10232. [Google Scholar] [CrossRef]
- Nofal, Z.M.; El-Zahar, M.I.; Abd El-Karim, S.S. Novel Coumarin Derivatives with Expected Biological Activity. Molecules 2000, 5, 99–113. [Google Scholar] [CrossRef]
- Kulkarni, M.; Kulkarni, G.; Lin, C.-H.; Sun, C.-M. Recent advances in coumarins and 1-azacoumarins as versatile biodynamic agents. Curr. Med. Chem. 2006, 13, 2795–2818. [Google Scholar] [CrossRef] [PubMed]
- Farkouth, M.; Greenberg, B. An evidence-based review of the cardiovascular risks of non-steroidal anti-inflammatory drugs. Am. J. Cardiol. 2009, 109, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Day, R.; Graham, G.; Williams, K. Propionic acid derivative drug (Profens). In Compendium of Inflammatory Diseases; Birkhäuser: Basel, Switzerland, 2013; pp. 1–6. [Google Scholar] [CrossRef]
- Davies, N. Clinical Pharmacokinetics of Flurbiprofen and its Enantiomers. Clin. Pharmacokinet. 1995, 28, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Manolov, S.; Ivanov, I.; Bojilov, D.; Nedialkov, P. Synthesis, In Vitro Anti-Inflammatory Activity, and HRMS Analysis of New Amphetamine Derivatives. Molecules 2023, 28, 151. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).