Hexakis(μ-3-aminopropanethiolato-1κ6N,S:2κ3S;3κ6N,S:2κ3S)cadmium(II)dirhodium(III) Dibromide Tetrahydrate
Abstract
1. Introduction
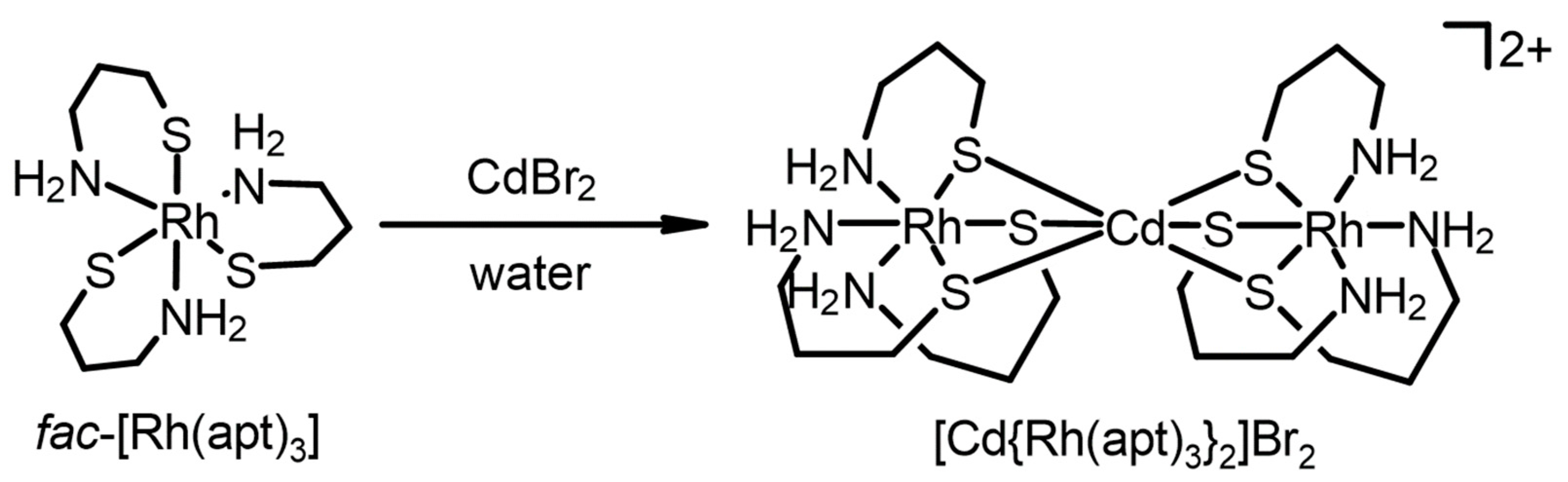
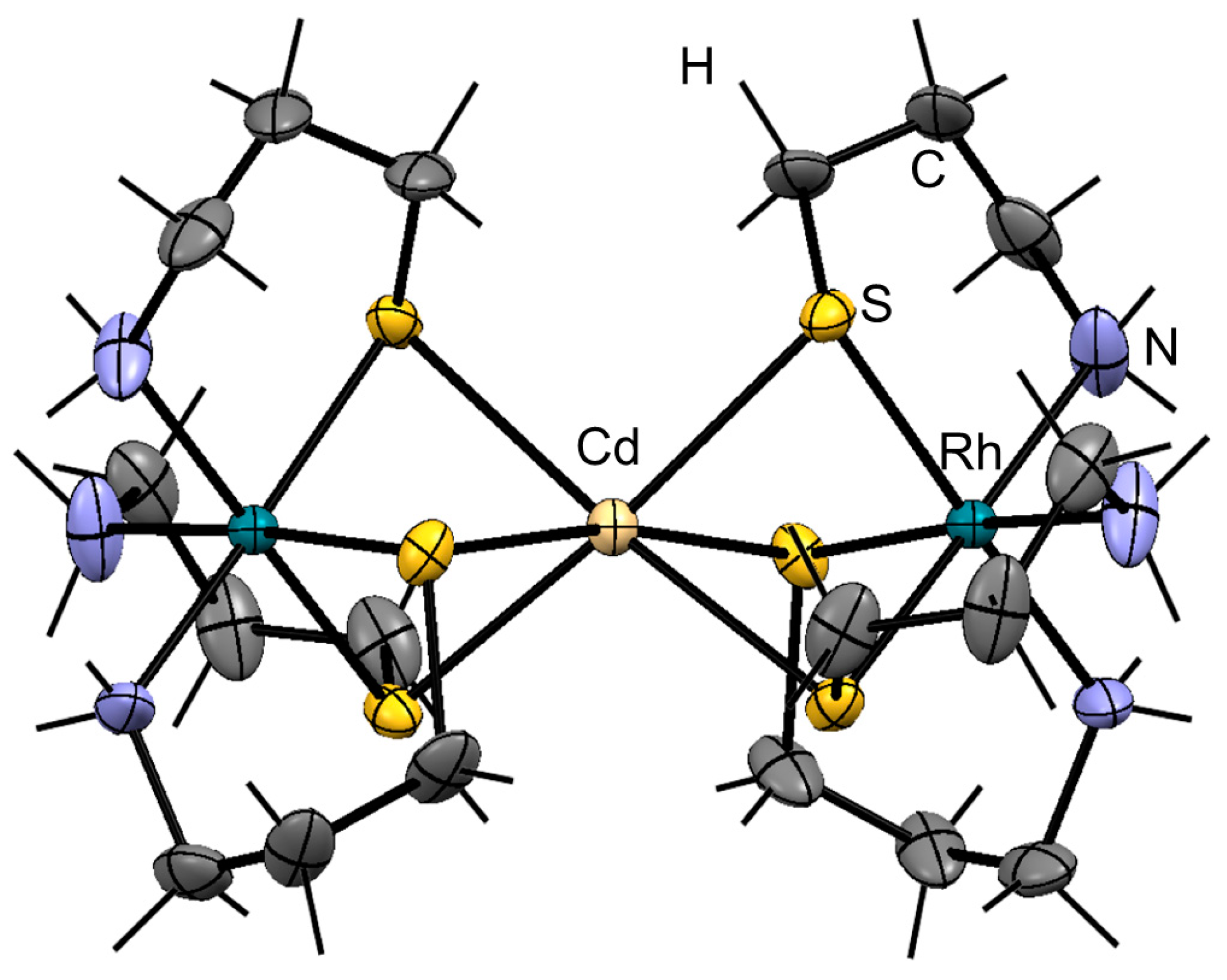
2. Results and Discussion
3. Materials and Methods
3.1. Physical Measurements
3.2. X-ray Crystal Structure Determination
3.3. Synthesis of [Cd{Rh(apt)3}2]Br2·4H2O
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henkel, G.; Krebs, B. Metallothioneins: Zinc, cadmium, mercury, and copper thiolates and selenolates mimicking protein active site features−structural aspects and biological implications. Chem. Rev. 2004, 104, 801–824. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, H. Structural chemistry of complexes of (n − 1)d10 nsm metal ions with β-N-donor substituted thiolate ligands (m = 0, 2). Coord. Chem. Rev. 2005, 249, 799–827. [Google Scholar] [CrossRef]
- Yamada, T.; Tsumita, M.; Hirano, A.; Miyashita, Y.; Fujisawa, K.; Okamoto, K. Syntheses, stereochemistry, reactivity, and some properties of sulfur-bridged cobalt(III)–cadmium(II) polynuclear complexes derived from mononuclear cobalt(III) complex with D-penicillaminate. Inorg. Chim. Acta 2002, 332, 108–114. [Google Scholar] [CrossRef]
- Konno, T. Aggregation of Octahedral Thiolato Complexes by Forming Sulfur-Bridged Structures with Transition Metal Ions. Bull. Chem. Soc. Jpn. 2004, 77, 627–649. [Google Scholar] [CrossRef]
- Kouno, M.; Kuwamura, N.; Yoshinari, N.; Konno, T. 3-Aminopropanethiol versus 2-Aminoethanethiol That Leads to Different S-Bridged Multinuclear Structures Composed of Rhodium(III) Octahedrons. Chem. Lett. 2017, 46, 1542–1545. [Google Scholar] [CrossRef]
- Kouno, M.; Yoshinari, N.; Kuwamura, N.; Yamagami, K.; Sekiyama, A.; Okumura, M.; Konno, T. Valence Interconversion of Octahedral Nickel(II/III/IV) Centers. Angew. Chem. Int. Ed. 2017, 56, 13762–13766. [Google Scholar] [CrossRef]
- Kouno, M.; Minami, K.; Kuwamura, N.; Konno, T. A Mixed-Valence Copper(I)-Copper(II) Core Supported by Rhodium(III) Octahedrons with 3-Aminopropanethiolate. Chem. Lett. 2019, 48, 122–125. [Google Scholar] [CrossRef]
- McCleverty, J.A.; Gill, S.; Kowalski, R.S.Z.; Bailey, N.A.; Adams, H.; Lumbard, K.W.; Murphy, M.A. Aspects of the inorganic chemistry of rubber vulcanisation. Part 3. Anionic cadmium complexes derived from dialkyldithiocarbamates, 2-mercaptobenzothiazole and its derivatives, and dialkyl dithiophosphates, and the crystal and molecular structures of [NBun4][Cd(S2CNEt2)3], [NEt4][Cd(C7H4NS2)3], and [NMe4][Cd{S2P(OPri)2}3]. J. Chem. Soc. Dalton Trans. 1982, 1982, 493–503. [Google Scholar]
- Baggio, R.; Frigerio, A.; Halac, E.B.; Vega, D.; Perec, M. Synthesis and characterization of dithiocarbonate derivatives of zinc and cadmium bis(dithiocarbamates). J. Chem. Soc. Dalton Trans. 1992, 1992, 1887–1892. [Google Scholar] [CrossRef]
- Tan, Y.S.; Sudlow, A.L.; Molloy, K.C.; Morishima, Y.; Fujisawa, K.; Jackson, W.J.; Henderson, W.; Halim, S.N.B.A.; Ng, S.W.; Tiekink, E.R.T. Supramolecular Isomerism in a Cadmium Bis(N-Hydroxyethyl, N-isopropyldithiocarbamate) Compound: Physiochemical Characterization of Ball (n = 2) and Chain (n = ∞) Forms of {Cd[S2CN(iPr)CH2CH2OH]2·solvent}n. Cryst. Growth Des. 2013, 13, 3046–3056. [Google Scholar] [CrossRef]
- Macreadie, L.K.; Forsyth, C.M.; Turner, D.R.; Chesman, A.S.R. Cadmium tris(dithiocarbamate) ionic liquids as single source, solvent-free cadmium sulfide precursors. Chem. Commun. 2018, 54, 8925–8928. [Google Scholar] [CrossRef]
- Glinskaya, L.A.; Zemskova, S.M.; Klevtsova, R.F.; Larionov, S.V.; Gromilov, S.A. The preparation, structures and thermal properties of [MEn3][Cd(S2CNEt2)3]2 [M = zinc(II), cadmium(II)] complexes. Polyhedron 1992, 11, 2951–2956. [Google Scholar] [CrossRef]
- Manar, K.K.; Yadav, M.K.; Anamika; Drew, M.G.B.; Singh, N. Influence of functionalities over polymer, trimer, dimer formation and optical properties of cadmium dithiocarbamates. Polyhedron 2016, 117, 592–599. [Google Scholar] [CrossRef]
- Tan, Y.S.; Halim, S.B.A.; Tiekink, E.R.T. Exploring the crystallization landscape of cadmium bis(N-hydroxyethyl, N-isopropyldithiocarbamate), Cd[S2CN(iPr)CH2CH2OH]2. Z. Krist. Cryst. Mater. 2016, 231, 113–126. [Google Scholar] [CrossRef]
- Sakane, G.; Kawasaki, H.; Yamasaki, M.; Adachi, H.; Shibahara, T. Sulfur-Bridged Cubane-Type Molybdenum-Cadmium Clusters with Diethyldithiophosphato or Nitrilotriacetato Ligands. Chem. Lett. 1999, 28, 631–632. [Google Scholar] [CrossRef]
- Sakane, G.; Kawasaki, H.; Oomori, T.; Yamasaki, M.; Adachi, H.; Shibahara, T. Cubane-Type Molybdenum-Zinc or -Cadmium Mixed-Metal Clusters with Diethyldithiophosphate or Nitrilotriacetate Ligands. J. Cluster Sci. 2002, 13, 75–102. [Google Scholar] [CrossRef]
- Glass, R.S.; Steffen, L.K.; Swanson, D.D.; Wilson, G.S.; de Gelder, R.; de Graaff, R.A.G.; Reedijk, J. Bis(trithiacyclononane)metal(II) compounds and Jahn-Teller distortions from octahedral geometry, electrochemistry, spectroscopy, and crystal structures of the copper bis(tetrafluoroborate) bis(acetonitrile) complex at 177 K and the cadmium bis(tetrafluoroborate) and copper bis(tetrafluoroborate) bis(nitromethane) complexes at 300 K. Inorg. Chim. Acta 1993, 207, 241–252. [Google Scholar]
- Helm, M.L.; Combs, C.M.; VanDerveer, D.G.; Grant, G.J. Homoleptic Group 12 metal complexes of macrocyclic thioethers: The crystal structures of bis(1,4,7-trithiacyclodecane )M(II) perchlorate: M(II) = zinc(II), cadmium(II), mercury(II). Inorg. Chim. Acta 2002, 338, 182–188. [Google Scholar] [CrossRef]
- Helm, M.L.; Loveday, K.D.; Combs, C.M.; Bentzen, E.L.; VanDerveer, D.G.; Rogers, R.D.; Grant, G.J. Heavy metal complexes of macrocyclic trithioethers. J. Chem.Cryst. 2003, 33, 447–455. [Google Scholar] [CrossRef]
- Helm, M.L.; Hill, L.L.; Lee, J.P.; Van Derveer, D.G.; Grant, G.J. Cadmium-113 NMR studies on homoleptic complexes containing thioether ligands: The crystal structures of [Cd([12]aneS4)2](ClO4)2, [Cd([18]aneS4N2)](PF6)2 and [Cd([9]aneS3)2](PF6)2. Dalton Trans. 2006, 2006, 3534–3543. [Google Scholar] [CrossRef]
- Stalhandske, C.M.V.; Stalhandske, C.I.; Sandstrom, M.; Persson, I. Crystal Structure of N,N-Dimethylthioformamide Solvates of the Divalent Group 12 Ions with Linear Coordination Geometry for Mercury(II), Tetrahedral for Zinc(II), and Octahedral for Cadmium(II). Inorg. Chem. 1997, 36, 3167–3173. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Mostafa, G.; Ghosh, A.; Laskar, I.R.; Chaudhuri, N.R. Construction of a unique three-dimensional array with cadmium(II). J. Chem. Soc. Dalton Trans. 1999, 1999, 9–10. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, Y.; Zhang, Y.; Li, B.; Zhang, Y. Synthesis, crystal structure and luminescent properties of a novel cadmium coordination polymer with unprecedented one-dimensional hetero-triple-stranded chain. Inorg. Chem. Commun. 2004, 7, 1181–1183. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, H.; Dang, D.; Shang, W.; Pan, X.J. Synthesis, crystal structure and luminescent properties of a thiocyanato-bridged two-dimensional heteronuclear polymeric complex of cadmium(II) and nickel(II). J. Mol. Struct. 2009, 934, 53–56. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, V.; Gupta, A.N.; Manar, K.K.; Drew, M.G.B.; Singh, N. Influence of ligand environments on the structures and luminescence properties of homoleptic cadmium(ii) pyridyl functionalized dithiocarbamates. CrystEngComm 2014, 16, 6765–6774. [Google Scholar] [CrossRef]
- Sun, Z.F.; Duan, C.Y.; You, X.Z. A mixed-ligand cadmium(II) complex of xanthic acid and N,N′-bis(4-methoxyphenyl)thiourea. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1994, 50, 1012–1014. [Google Scholar] [CrossRef]
- Petrova, R.; Angelova, O.; Macícek, J. Molecular Adducts of Inorganic Salts. VII. Cadmium Tetraoxorhenium Hexakis(thiourea) Hydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1997, 53, 565–568. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Takemoto, M.; Osaka, K.; Nishibori, E.; Moriyoshi, C.; Kubota, Y.; Kuroiwa, Y.; Sugimoto, K. High-throughput powder diffraction measurement system consisting of multiple MYTHEN detectors at beamline BL02B2 of SPring-8. Rev. Sci. Instrum. 2017, 88, 085111. [Google Scholar] [CrossRef]
- Sheldrick, G.M.A. A short history of SHELX. Acta. Cryst. 2008, A64, 112–113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouno, M.; Yoshinari, N.; Kojima, T.; Konno, T. Hexakis(μ-3-aminopropanethiolato-1κ6N,S:2κ3S;3κ6N,S:2κ3S)cadmium(II)dirhodium(III) Dibromide Tetrahydrate. Molbank 2023, 2023, M1684. https://doi.org/10.3390/M1684
Kouno M, Yoshinari N, Kojima T, Konno T. Hexakis(μ-3-aminopropanethiolato-1κ6N,S:2κ3S;3κ6N,S:2κ3S)cadmium(II)dirhodium(III) Dibromide Tetrahydrate. Molbank. 2023; 2023(3):M1684. https://doi.org/10.3390/M1684
Chicago/Turabian StyleKouno, Masahiro, Nobuto Yoshinari, Tatsuhiro Kojima, and Takumi Konno. 2023. "Hexakis(μ-3-aminopropanethiolato-1κ6N,S:2κ3S;3κ6N,S:2κ3S)cadmium(II)dirhodium(III) Dibromide Tetrahydrate" Molbank 2023, no. 3: M1684. https://doi.org/10.3390/M1684
APA StyleKouno, M., Yoshinari, N., Kojima, T., & Konno, T. (2023). Hexakis(μ-3-aminopropanethiolato-1κ6N,S:2κ3S;3κ6N,S:2κ3S)cadmium(II)dirhodium(III) Dibromide Tetrahydrate. Molbank, 2023(3), M1684. https://doi.org/10.3390/M1684






