(E)-N-(3-(5-(3-Acetamidopropyl)-3,6-dioxopiperazin-2-yl)propyl)-5-hydroxy-3-methylpent-2-enamide
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Isolation and Cultivation of the Fungus
3.2. Isolation and Structural Characterisation of Albrnazine PS (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lomovskaya, N.; Otten, S.L.; Doi-Katayama, Y.; Fonstein, L.; Liu, X.C.; Takatsu, T.; Inventi-Solari, A.; Filippini, S.; Torti, F.; Colombo, A.L.; et al. Doxorubicin Overproduction in Streptomyces peucetius: Cloning and Characterization of the dnrU Ketoreductase and dnrV Genes and the doxA Cytochrome P-450 Hydroxylase Gene. J. Bacteriol. 1999, 181, 305–318. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.C.L.; Paulo, A.J.; de Albuquerque Lima, C.; de Lima Filho, J.L.; Souza-Motta, C.M.; Vidal, E.E.; Nascimento, T.P.; de Araújo Viana Marques, D.; Porto, A.L.F. Lovastatin Producing by Wild Strain of Aspergillus terreus Isolated from Brazil. Prep. Biochem. Biotechnol. 2021, 51, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Silva, B.; Rainey, F.A.; Warren-Rhodes, K.A.; McKay, C.P.; Navarro-González, R. Atacama Desert Soil Microbiology. In Microbiology of Extreme Soils; Dion, P., Nautiyal, C.S., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 13. [Google Scholar] [CrossRef]
- Azua-Bustos, A.; González-Silva, C.; Corsini, G. The Hyperarid Core of the Atacama Desert, an Extremely Dry and Carbon Deprived Habitat of Potential Interest for the Field of Carbon Science. Front. Microbiol. 2017, 8, 993. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R.; Jaspars, M. Natural Product Diversity of Actinobacteria in the Atacama Desert. Antonie Van Leeuwenhoek 2018, 111, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Carro, L.; Razmilic, V.; Nouioui, I.; Richardson, L.; Pan, C.; Golinska, P.; Asenjo, J.A.; Bull, A.T.; Klenk, H.P.; Goodfellow, M. Hunting for Cultivable Micromonospora Strains in Soils of the Atacama Desert. Antonie Van Leeuwenhoek 2018, 111, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
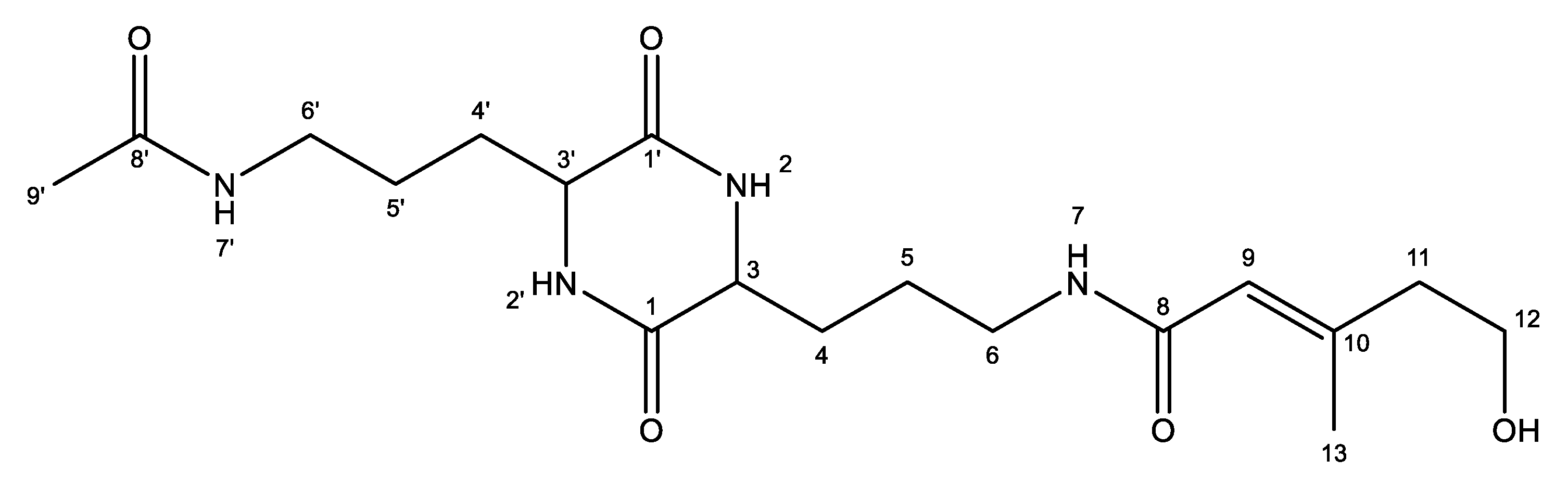


| Position a | δC (ppm) | δH (ppm), Multiplicity (J in Hz) | COSY a | HMBC a (H→C) |
|---|---|---|---|---|
| 1/1’ | 168.3 C | - | - | - |
| 2/2’ | - | 8.13 s | 3/3’ | 1/1’, 3’/3 |
| 3/3’ | 54.1 CH | 3.80 t (5.1) | 2/2’, 4/4’ | 1’/1, 4/4’, 5/5’ |
| 4/4’ | 31.0 CH2 | 1.65 m (2H) | 3/3’, 5/5’ | 3/3’ |
| 5/5’ | 25.0 CH2 | 1.43 m (2H) | 4/4’, 6/6’ | 4/4’ |
| 6 | 38.3 CH2 | 3.04 m (2H) | 5, 7 | 4, 5, 8 |
| 7 | - | 7.75 t (5.2) | 6 | 8 |
| 8 | 166.5 C | - | - | - |
| 9 | 120.3 CH | 5.62 s | 11, 13 | 8, 10, 11, 13 |
| 10 | 149.8 C | - | - | - |
| 11 | 43.8 CH2 | 2.17 t (6.8, 2H) | 12 | 9, 10, 12, 13 |
| 12 | 59.3 CH2 | 3.51 t (6.8, 2H) | 12-OH, 11 | 10 |
| 12-OH | - | 4.54 s | 12 | - |
| 13 | 17.9 CH3 | 2.07 s (3H) | 9 | 8, 9, 10, 11 |
| 6’ | 38.3 CH2 | 2.99 m (2H) | 5’, 7’ | 4’, 5’, 8’ |
| 7’ | - | 7.81 t (5.1) | 6’ | 8’ |
| 8’ | 169.5 C | - | - | - |
| 9’ | 22.8 CH3 | 1.78 s (3H) | - | 8’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preet, G.; Astakala, R.V.; Rajakulendran, J.E.; Oluwabusola, E.T.; Ebel, R.; Jaspars, M. (E)-N-(3-(5-(3-Acetamidopropyl)-3,6-dioxopiperazin-2-yl)propyl)-5-hydroxy-3-methylpent-2-enamide. Molbank 2023, 2023, M1680. https://doi.org/10.3390/M1680
Preet G, Astakala RV, Rajakulendran JE, Oluwabusola ET, Ebel R, Jaspars M. (E)-N-(3-(5-(3-Acetamidopropyl)-3,6-dioxopiperazin-2-yl)propyl)-5-hydroxy-3-methylpent-2-enamide. Molbank. 2023; 2023(3):M1680. https://doi.org/10.3390/M1680
Chicago/Turabian StylePreet, Gagan, Rishi Vachaspathy Astakala, Joy Ebenezer Rajakulendran, Emmanuel T. Oluwabusola, Rainer Ebel, and Marcel Jaspars. 2023. "(E)-N-(3-(5-(3-Acetamidopropyl)-3,6-dioxopiperazin-2-yl)propyl)-5-hydroxy-3-methylpent-2-enamide" Molbank 2023, no. 3: M1680. https://doi.org/10.3390/M1680
APA StylePreet, G., Astakala, R. V., Rajakulendran, J. E., Oluwabusola, E. T., Ebel, R., & Jaspars, M. (2023). (E)-N-(3-(5-(3-Acetamidopropyl)-3,6-dioxopiperazin-2-yl)propyl)-5-hydroxy-3-methylpent-2-enamide. Molbank, 2023(3), M1680. https://doi.org/10.3390/M1680






