Abstract
The title compound, (±)-N-(1,2-bis(3,4-dimethoxyphenyl)ethyl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide, was obtained for the first time from 1,2-bis(3,4-dimethoxyphenyl) ethan-1-amine and (±)-flurbiprofen in one step. The newly synthesized bio-functional hybrid compound was fully characterized using 1H, 13C-NMR, UV, and mass spectral data.
1. Introduction
1,2-Bis(3,4-dimethoxyphenyl)ethan-1-amine (Figure 1) is a starting material for a wide range of biologically active naturally occurring alkaloids like 3-arylisoquinolines, benzo[c]phenanthridines, and protoberberines [1,2].

Figure 1.
Structural formula of 1,2-bis(3,4-dimethoxyphenyl)ethan-1-amine and flurbiprofen.
Pavines and isopavines are two relatively small subgroups of the isoquinoline alkaloids [3].
Numerous research teams have become interested in the isopavines over the last few decades due to their intriguing structures and possible medical uses. As a result, several elegant syntheses have been created [4,5,6,7].
It has been established that isopavines exhibit a variety of pharmaceutical effects. Along with having strong opioid receptor binding activity, the isopavine alkaloids and some of their synthetic analogues have been utilized to treat diseases of the nervous system such as Alzheimer’s, Parkinson’s disease, Huntington’s chorea, amyotrophic lateral sclerosis, and Down’s syndrome [4]. Representatives of natural isopavine alkaloids can be seen in Figure 2. In the blue colour is given the skeleton of 1,2-diphenylethan-1-amine, unifying the two natural representatives (−)-amurensine and (−)-reframidine with 1,2-bis(3,4-dimethoxyphenyl)ethan-1-amine.
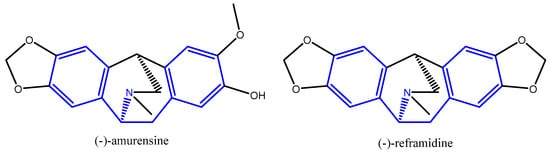
Figure 2.
Structural formulas of (−)-amurensine and (−)-reframidine.
Flurbiprofen (Figure 1) is a member of the phenylalkanoic acid series with the CAS Registry number of 86-55-5 and molecular formula of C15H13FO2. Flurbiprofen is classified as a Biopharmaceutical Classification System Type II drug [8,9,10]. It has analgesic, antipyretic, and anti-inflammatory properties and is commonly used to treat rheumatoid arthritis as well as migraines, osteoarthritis, and sore throat discomfort.
Fluorine has been widely employed to customize the biological behaviour of molecules and particles for the detection of major illnesses, to improve medicine therapeutic efficacy, and to allow cell tracking [11]. Medicinal chemists frequently include a fluorine atom into different pharmaceutical compounds to increase selectivity, potential, physicochemical qualities, and other attributes. The introduction of fluorine atoms has showed the potential to affect various essential features such as metabolic pathways, pharmacokinetics, and permeability [12]. In this respect, there has been a rise in the development of a number of novel fluorine-containing compounds in recent years, as well as modifications to existing ones. These chemicals are extremely interesting in terms of understanding their biological features and potential future use in pharmacy [13].
The synthesis of amides is particularly essential due to the prevalence of this theme in biological systems as well as in the pharmaceutical business, where amide manufacturing is believed to be the most frequent chemical reaction used. Amide groups are also synthetically flexible and capable of participating in a broad variety of transformations. As a result, we were eager to create a novel hybrid molecule composed of a flurbiprofen fragment and the structure of 1,2-bis(3,4-dimethoxyphenyl)ethan-1-amine. This novel molecule is also intriguing because of the possibilities that can be discovered when employing it as a starting material for the manufacturing of numerous natural derivatives.
2. Results and Discussion
In this paper, we report the synthesis of (±)-N-(1,2-bis(3,4-dimethoxyphenyl)ethyl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide 3 (Scheme 1).
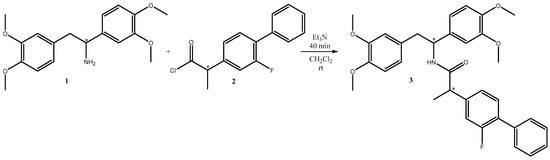
Scheme 1.
Synthesis of N-(1,2-bis(3,4-dimethoxyphenyl)ethyl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide 3.
For this purpose, 2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanoyl chloride 2 (1 mmol) was added to the solution of 1,2-bis(3,4-dimethoxyphenyl)ethan-1-amine 1 (1 mmol) in dichloromethane. A slight excess of trimethylamine (1.5 mmol) was carefully added after stirring the reaction mixture for 10 min. TLC monitoring (Et2O) showed the consumption of the starting material in 30 min. As we used a racemic mixture of compounds 1 and 2, four stereoisomers were obtained, which appeared as a single spot on TLC (Rf = 0.54 in diethyil ether) and a single band over the HPLC chromatogram (Rt = 4.73 min, Figure S6 in Supplementary Materials). The proximity of the two chiral centres is a reasonable explanation of the observed chromatographic behaviour with the four stereoisomers.
As we have a mixture of diastereoisomers, the signals for all 68 protons can be seen quite clearly when looking at the new hybrid’s 1H NMR spectrum data. In the aliphatic region, two sets of doublets at 1.25 ppm and 1.29 ppm are found and can be assigned to methyl groups of flurbiprofen residues. In the 1H NMR spectrum of compound 3 (Figure S1), the presence of a 1:1 mixture of a couple of diastereomers was readily detectable. In the aliphatic region, two sets of doublets at 1.25 and 1.29 ppm were found and could be assigned to methyl groups of flurbiprofen residues. The benzylic CH2s were found in the range 2.99–3.76 ppm. Eight methoxy groups appeared as singlets in the range 3.58–3.77 ppm, where the CHs belonging to flurbiprofen moieties also resonated. The NHs appeared around 8.45 ppm, whereas their vicinal CHs gave signals in the range 4.95–5.03 ppm. All 28 aromatic protons resonated in the range 6.63–7.55 ppm. The 13C NMR spectrum confirmed the structure of the newly synthesized molecule 3.
A thorough mass spectrum investigation of the new hybrid was also carried out (Scheme 2).
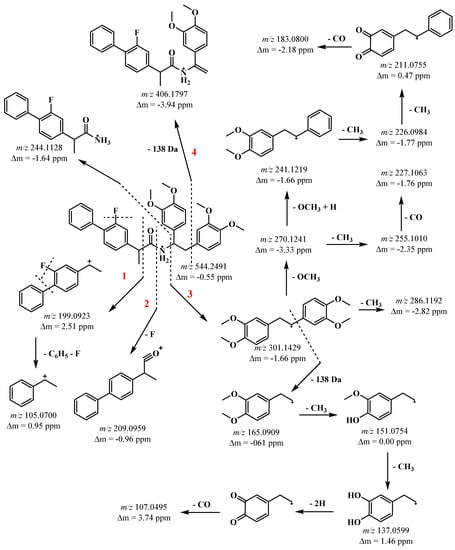
Scheme 2.
Proposed fragmentation of protonated compound 3.
Under MS/MS conditions, compound 3 underwent cleavage of the amide bond, yielding fragments characteristic of flurbiprofen and the 1,2-bis(3,4-dimethoxyphenyl) ethan-1-ylium cation. Cleavage of C-C(=O) (path 1), N-C(=O) (path 2), and N-C (path 3) bonds yielded ions that characterized flurbiprofen m/z 199, m/z 209, and m/z 244 (Scheme 2), respectively. According to our prior research, an m/z 199 ion is typical, which, under ESI/MS-MS conditions, loses a F atom and a phenyl fragment, yielding an m/z 105 ion (path 1) (Scheme 2). The N-C (path 3) link was cleaved, yielding two fragments: 244 and the distinctive 1,2-bis(3,4-dimethoxyphenyl)ethan-1-ylium cation with m/z 301 (Scheme 2, Figure S5). Parallel fragmentation of the same ion results in the loss of 138 Da, CH3 radical (m/z 286), and OCH3 groups. The ion m/z 165 is formed when a 1,2-bis(3,4-dimethoxyphenyl)ethan-1-ylium cation (m/z 301) loses a 1,2-dimethoxybenzene fragment (138 Da) (Scheme 2, Figure S5). The resultant ion loses CH3 radicals sequentially, generating the m/z 151 and m/z 137 ions. The m/z 137 product ion undergoes rearrangement with the release of a neutral CO molecule to generate the m/z 107 ion. The ion m/z 241 was created by sequentially losing OCH3 groups (Scheme 2, Figure S5). Furthermore, it gradually loses CH3 radicals, yielding a quinoid ion with m/z 211. A neutral molecule is lost using the quinoid ion, resulting in an m/z 183 ion. Path 4 results in the m/z 406 ion, which is likewise related to a 138 Da loss (Scheme 2, Figure S5).
The structure of the newly obtained hybrid 3 is proven based on all the experimental data.
3. Materials and Methods
All reagents and chemicals were obtained from commercial suppliers (Sigma-Aldrich S.A. and Riedel-de Haën, Sofia, Bulgaria) and used as received. The NMR spectral measurements were collected using a Bruker Avance Neo 400 spectrometer (BAS-IOCCP—Sofia, Bruker, Billerica, MA, USA). 1H NMR and 13C NMR spectra for compound 3 were taken in DMSO-d6 at 400 MHz and 101 MHz, respectively. Chemical shifts are given in relative ppm and were referenced to tetramethylsilane (TMS) (δ = 0.00 ppm) as an internal standard; the coupling constants are indicated in Hz. At room temperature, the NMR spectra were recorded (ac. 295 K). The melting point was determined on a Boetius hot stage apparatus and is uncorrected. Absorbance was measured with a spectrophotometer Camspec M508, Leeds, UK. The MS analysis was performed on a Q Exactive Plus high-resolution mass spectrometer (HRMS) with a heated electrospray ionization source (HESI-II) (Thermo Fisher Scientific, Inc., Bremen, Germany) equipped with a Dionex Ultimate 3000RSLC ultrahigh-performance liquid chromatography (UHPLC) system (Thermo Fisher Scientific, Inc., Waltham, MA, USA). TLC was carried out on precoated 0.2 mm Fluka silica gel 60 plates (Merck KGaA, Darmstadt, Germany). For the HPLC analysis, chromatographic-grade methanol was used (VWR, Vienna, Austria). A Millipore purifier (Millipore, Burlington, MA, USA) was used to obtain water for HPLC. The equipment used for the HPLC analysis consisted of a quaternary mixer Smartline Manager 5000, pump Smartline 1000, and PDA 2800 detector (Knauer, Berlin, Germany). The column used was a Purospher C18, 250 × 4.6 mm i.d., 5 µm particle size (Merck, Darmstadt, Germany). The chromatographic separation was carried out using methanol/water = 90/10. The compound was monitored at 254 nm and 280 nm.
3.1. (±)-(2-(2-Fluoro-[1,1′-biphenyl]-4-yl)propanoyl Chloride 2
An excess of thionyl chloride (1.2 mmol, 0.087 mL) was added to (±)-flurbiprofen (1.0 mmol, 0.244 g) dissolved in toluene (30 mL). For 2 h, the reaction mixture was stirred under reflux. The excess of thionyl chloride and toluene was removed under reduced pressure. The obtained 2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanoyl chloride 2 was used without further purification.
3.2. Synthesis of (±)-N-(1,2-Bis(3,4-dimethoxyphenyl)ethyl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide 3
To a solution of 1,2-bis(3,4-dimethoxyphenyl)ethan-1-amine 2 (1.0 mmol, 0.317 g) in dichloromethane (30 mL), an equal amount of 2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanoyl chloride 2 (1 mmol, 0.262 g) was added. After 10 min, triethylamine (1.2 mmol, 1.67 mL) was added to the solution. After 30 min, the reaction mixture was washed with diluted hydrochloric acid, a saturated Na2CO3 solution, and brine. The solvent was dried over anhydrous Na2SO4 and then removed under reduced pressure. The new hybrid molecule was purified with filtration through short-column chromatography over neutral Al2O3 (CH2Cl2).
(±)-N-(1,2-Bis(3,4-dimethoxyphenyl)ethyl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propenamide 3
White solid (m.p. 184–186 °C), yield 93% (0.507 g), Rf = 0.54 (diethyl ether), 1H NMR (400 MHz, DMSO-d6) δ 8.45 (complex signal, 2H, 2 x NH), 7.55 (dq, J = 7.7, 1.5 Hz, 2H), 7.49 (td, J = 3.4, 2.9, 1.6 Hz, 4H), 7.45–7.36 (m, 4H), 7.36–7.31 (m, 1H), 7.19–7.14 (m, 2H), 7.06 (dd, J = 12.3, 1.8 Hz, 1H), 7.01–6.97 (m, 2H), 6.93–6.88 (m, 2H), 6.87–6.82 (m, 4H), 6.81 (s, 1H), 6.79–6.73 (m, 3H), 6.71–6.63 (m, 2H), 5.03–4.95 (complex signal, 2H, 2 x CHN), 3.77 (s, 3H), 3.74 (s, 3H), 3.73 (s, 3H), 3.71 (s, 3H), 3.71–3.69 (complex signal, 2H, 2 x CHCH3), 3.69 (s, 3H), 3.62 (s, 3H), 3.59 (s, 3H), 3.58 (s, 3H), 2.99–2.76 (complex signal, 4H, 2 x CH2), 1.29 (d, J = 7.1 Hz, 3H), 1.25 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 172.20/172.16 (C=O), 159.20 (d, 1JC-F = 245.6 Hz), 157.97 (Ar), 149.04/148.92 (Ar), 148.74/148.66 (Ar), 148.11/147.99 (Ar), 147.66/147.56 (Ar), 144.53 (Ar), 136.58/136.21 (Ar), 135.43/131.53 (Ar), 130.82/130.79 (Ar), 130.62 (d, 3JC-F = 3.8 Hz), 129.16/129.13 (Ar), 129.06/129.03 (Ar), 128.18/128.13 (Ar), 126.81/126.68 (Ar), 124.22/124.05 (Ar), 121.66/121.45 (Ar), 118.92/118.87 (Ar), 115.30 (d, 2JC-F = 23.2 Hz), 115.14/114.91 (Ar), 113.61/113.41 (Ar), 112.08/111.98 (Ar), 111.92/111.70 (Ar), 111.02/110.64 (Ar), 56.02/55.96 (OCH3), 55.91 (OCH3), 55.83/55.67 (OCH3), 55.59/54.10 (OCH3), 54.06 (CH-N), 45.05/44.96 (CH-CH3), 42.53/42.46 (C(Ar)-CH2CH), 18.70/18.58 (CHCH3). UV λmax, MeOH: 257 (ε = 23400) nm, 300 (ε = 8560) nm. HRMS Electrospray ionization (ESI) m/z calcd for [M+H]+ C33H35FNO5+ = 544.2494, found 544.2491 (mass error ∆m = −0.55 ppm), calcd for [M+Na]+ C33H34FNO5Na+ = 566.2313, found 566.2307 (mass error ∆m = −1.06 ppm).
Supplementary Materials
The following supporting information can be downloaded online, Figure S1: 1H-NMR spectrum of compound 3; Figure S2: 13C-NMR spectrum of compound 3; Figure S3: UV spectrum of compound 3; Figure S4: ESI-HRMS of compound 3; Figure S5: Mass spectrum of 3 obtained by positive ion ESI-MS/MS; Figure S6: HPLC chromatogram of compound 3.
Author Contributions
Conceptualization, I.I. and S.M.; methodology, S.M.; software, S.M.; validation, D.B., S.M. and I.I.; formal analysis, S.M., D.B. and P.N.; investigation, S.M. and D.B.; resources, I.I.; data curation, S.M.; writing—original draft preparation, S.M. and D.B.; writing—review and editing, S.M., D.B. and I.I.; visualization, S.M.; supervision, I.I.; project administration, S.M.; funding acquisition, I.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Plovdiv, grant number: ΦΠ23-XΦ-005.
Data Availability Statement
The data presented in this study are available in this article and supporting Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tellitu, I.; Badía, D.; Domínguez, E.; Carrillo, L. A convenient access to protoberberine derivatives. Heterocycles 1996, 10, 2099–2112. [Google Scholar] [CrossRef]
- Badía, D.; Domíngues, E.; Tellitu, I. Silicon-mediated isoquinoline synthesis: Preparation and stereochemical characterization of 4-hydroxy-3-phenylisoquinolines. Tetrahedron 1992, 48, 4419–4430. [Google Scholar] [CrossRef]
- Gözler, B. Chapter 7. The Alkaloids. In Pavine and Isopavine Alkaloids; Academic Press, Inc.: Cambridge, MA, USA, 1987; Volume 31. [Google Scholar]
- Sun, L.; Li, D.; Zhou, X.; Zhang, D.; Wang, J.; He, Z.; Jiang, R.; Chen, W. General and catalytic enantioselective approach to isopavine alkaloids. J. Org. Chem. 2017, 82, 12899–12907. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.-Y.; Li, Z.; Li, L.; Ma, Y.; Hu, S.; Wu, Z. Concise enantioselective total synthesis of isopavine alkaloids. J. Org. Chem. 2023, 88, 4317–4324. [Google Scholar] [CrossRef] [PubMed]
- Sunderhaus, J.; Dockendorff, C.; Martin, S. Synthesis of diverse heterocyclic scaffolds via tandem additions to imine derivatives and ring-forming reactions. Tetrahedron 2009, 65, 6454–6469. [Google Scholar] [CrossRef] [PubMed]
- Dragoli, D.; Burdett, M.; Ellman, J. Design, synthesis, and utility of a support-bound tert-butanesulfinamide. J. Am. Chem. Soc. 2001, 123, 10127–10128. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, T.; Dong, L.; Wang, X.; Huo, Q.; Wang, H.; Jiang, Z.; Shan, X.; Pan, W.; Yang, X. Design and evaluation of bilayer pump tabler of flurbiprofen solid dispersion for zero-order controlled delivery. J. Pharm. Sci. 2018, 107, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Park, Y.-J.; Kang, J.; Yong, C.; Choi, H.-G. Physicochemical characterization and in vivo evaluation of flurbiprofen-loaded solid dispersion without crystalline change. Drug Deliv. 2011, 18, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Mantas, A.; Labbe, V.; Loryan, I.; Mihranyan, A. Amorphisation of free acid ibuprofen and other profens in mixtures with nanocellulose: Dry powder formulation strategy for enhanced solubility. Pharmaceutics 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, K.; Fu, C.; Peng, H.; Hawker, C.; Whittaker, A. Biological Utility of Fluorinated compounds: From Materials Design to Molecular Imaging, Therapeutics and Environmental Remediation. Chem. Rev. 2022, 122, 167–208. [Google Scholar] [CrossRef] [PubMed]
- Caron, S. Where does the fluorine come from? A review on the challenges associated with the synthesis of organofluorine compounds. Org. Process Res. Dev. 2020, 24, 470–480. [Google Scholar] [CrossRef]
- Mlostoń, G.; Shermolovich, Y.; Heimgartner, H. Synthesis of fluorinated and fluoroalkylated heterocycles containing at least one sulfur atom via cycloaddition reactions. Meterials 2022, 15, 7244. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).