Abstract
2-Substituted 1,4-benzodioxanes bearing one or more substituents at benzene are important templates in the design and synthesis of a large variety of biologically active compounds. One of the most straightforward synthetic strategies to prepare them in racemic form and with a 2-substituent susceptible to further synthetically useful conversions is the condensation of commercially available methyl 2,3-dibromopropionate with already suitably functionalized catechol. Here, we obtain methyl 8- and 5-nitro-1,4-benzodioxane-2-carboxylate by reaction of methyl 2,3-dibromopropionate with 3-nitrocatechol. After separation, the two positional isomers could be unequivocally identified by HMBC NMR analysis.
1. Introduction
1,4-Benzodioxane has been and is extensively used as a base scaffold to design a large variety of biologically active compounds [1,2,3,4]. Substitution at C(2) makes this substructure chiral (Figure 1), and high eudismic ratios are frequently associated with bioactive enantiomeric 2-substituted 1,4-benzodioxanes [1]. 2-Carboxy, 2-hydroxymethyl and 2-aminomethyl-1,4-benzodioxane are their most accessible and versatile synthetic precursors [5]. Indeed, many different preparative approaches have been developed to obtain them in unichiral form, ranging from enantioselective syntheses to any kind of racemate resolution and use of starting C3 synthetic units available from the “chiral pool” [5]. On the other hand, decoration of the benzene ring (Figure 1), a feature of benzodioxane derivatives often critically related to selectivity for biological receptor subtypes or agonist/antagonist profile [6,7], can only be accomplished using two alternative strategies: condensation of a C3 synthetic unit with benzene already bearing the desired substituents or the introduction of the substituents of benzene into the preformed 2-substituted 1,4-benzodioxane. The two strategies are well exemplified by the recently reported condensation of ethyl 2,3-dibromopropionate with 3-methoxycatechol to give ethyl 5- and 8-methoxy-1,4-benzodioxane-2-carboxylate and Friedel–Craft acetylation of ethyl 1,4-benzodioxane-2-carboxylate to give ethyl 6- and 7-acetyl-1,4-benzodioxane-2-carboxylate [8]. Both the strategies suffer drawbacks in terms of the desired regioisomer’s yield and separation, which can be avoided but using purposely prepared polysubstituted benzene intermediates, generally O-mono-protected cathecol derivatives, such as, for instance, the regioisomers of nitrocatechol monobenzyl ether [6,9].
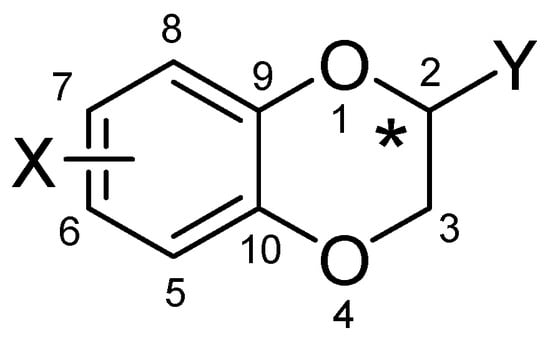
Figure 1.
General formula of a chiral 2-substituted 1,4-benzodioxane bearing a substituent at the benzene ring.
Weighing up all these issues related to substitutions at dioxane C(2) and free positions of benzene, condensation of largely available methyl 2,3-dibromopropionate and 3-substituted catechol seems the simplest approach to directly obtain 2-carboxy substituted 1,4-benzodioxanes bearing a further substituent at C(8) or C(5), with the only unknowns being the two regioisomers’ separability and certain identification (Scheme 1).
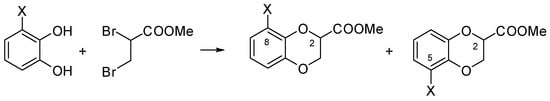
Scheme 1.
Synthesis of 8- and 5-X substituted methyl 1,4-benzodioxane-2-carboxylates from 3-X substituted catechol and methyl 2,3-dibromopropionate.
Recently, we have reported the unambiguous identification using HSQC and HMBC NMR analyses of methyl 8- and 5-bromo-1,4-benzodioxane-2-carboxylate, 1 and 2 respectively (Figure 2), obtained from methyl 2,3-dibromopropionate and 3-bromocatechol and then chromatographically separated, postulating that structure elucidation of other pairs of 2-substituted 1,4-benzodioxanes bearing an X substituent at the 8 or 5 position would be possible using the same procedure [10]. Here, as a continuation and confirmation of such an approach, we report the preparation of methyl 8- and 5-nitro-1,4-benzodioxane-2-carboxylate, 3 and 4 respectively (Figure 2), from methyl 2,3-dibromopropionate and 3-nitrocatechol and the assignment of the respective structures by analogous analysis of the different patterns of three-bond C-H correlations in the HMBC NMR spectrum.
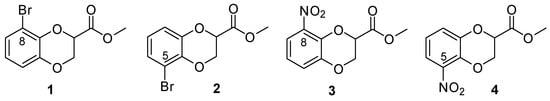
Figure 2.
Methyl 8- and 5-bromo-1,4-benzodioxane-2-carboxylates (1 and 2) and methyl 8- and 5-nitro-1,4-benzodioxane-2-carboxylates (3 and 4).
2. Results and Discussion
Attempts to prepare 3 and 4 by the same procedure previously reported for 1 and 2 [10] were unsuccessful. Indeed, the reaction between methyl 2,3-dibromopropionate and 3-nitrocatechol in boiling acetone in the presence of potassium carbonate gave minimal quantities of the desired products. Therefore, we planned the alternative use of an organic base in a higher boiling solvent. Equimolar amounts of commercially available methyl 2,3-dibromopropionate and 3-nitrocatechol, prepared by nitration of catechol as previously reported [11], were combined in acetonitrile in the presence of diisopropylethylamine and the mixture was heated at reflux temperature overnight. A standard workup provided a mixture of the two regioisomers, with predominance of the isomer quickly moving up the TLC silica gel plate and later identified as that nitrated at C(8) (3). This was recovered from the mixture as an insoluble solid by treatment with diethyl ether. Chromatography of the filtrate provided the residual part of 3 and, as a second eluted oily product, the compound was successively identified as 5-nitrated (4). The overall yield of 3 was 31% and that of 4 was 20%.
1H NMR and 13C NMR spectra of both 3 and 4 were consistent with the structure of the methyl ester of 1,4-benzodioxane-2-carboxylic acid nitrated at C(5) or C(8), namely, the expected products of the reaction (Figure 3). In the aliphatic region of the 1H NMR spectrum, two double doublets of dioxane CH2 and, at lower field, the pseudo triplet or double doublet of dioxane CH were recognizable, while, in the aromatic region, the triplet and the two double doublets, from high to low field, could be respectively ascribed to the hydrogen meta to the nitro group and the two hydrogens para and ortho to the nitro group. However, identical patterns of signals and multiplicity with non-decisive chemical shift differences hindered the assignment of the 3 and 4 structures to the first and the second eluted products, respectively, or vice versa. Moreover, the 13C NMR spectra were almost indistinguishable. HSQC NMR further confirmed the structures of 5- or 8-nitrated methyl 1,4-benzodioxane-2-carboxylates, allowing the assignment of the three signals between 118 and 122 ppm, from high to low field, to the three benzene CH ortho, meta and para positioned to NO2, respectively, and of the three peaks around 140 ppm to the other three benzene carbons not bearing hydrogen. As for the previously reported regioisomeric brominated benzodioxanes 1 and 2, HMBC NMR analysis was again resolutive for the identification of the signals imputable to C(9) and C(10), the two carbons shared between the two cycles, in 3 and 4 and consequently for the unequivocal identification of the two regioisomers. In the first eluted solid isomer 3, both the hydrogen meta to NO2, identified by the triplet, and the methylene hydrogens of dioxane showed a strong 3J H/C correlation to carbon signaling at 145 ppm. Therefore, this latter was the signal of C(10) and 3 was the 8-nitrated regioisomer (Figure 4). In 4, the second eluted oily regioisomer nitrated at C(5), the two above correlations took place with two different carbons: the hydrogen meta to NO2 with the carbon signaling at 144 ppm, which is C(9), and the dioxane methylene hydrogens with the carbon signaling at 138 ppm, which is C(10) (Figure 5). These results unequivocally indicated that 3, the first eluted product, was the isomer nitrated in the 8 position and that 4, the second eluted product, was the 5-nitrated isomer. The same sequence had been previously observed for 1, the 8-bromo isomer, and 2, the 5-bromo isomer [10]. As a consequence of such identification using HMBC NMR, the resonance position of the proton para to NO2 can now be assumed as confidently indicative of the NO2 position on methyl 1,4-benzodioxane-2-carboxylate: at higher field when NO2 is at C(8) and the carboxymethyl farther from the para proton, at lower field when NO2 is at C(5) and the carboxymethyl is closer to the para proton (Figure 4 and Figure 5). An analogous difference in the chemical shift was previously observed for the proton para to bromine in 1 and 2 [10].
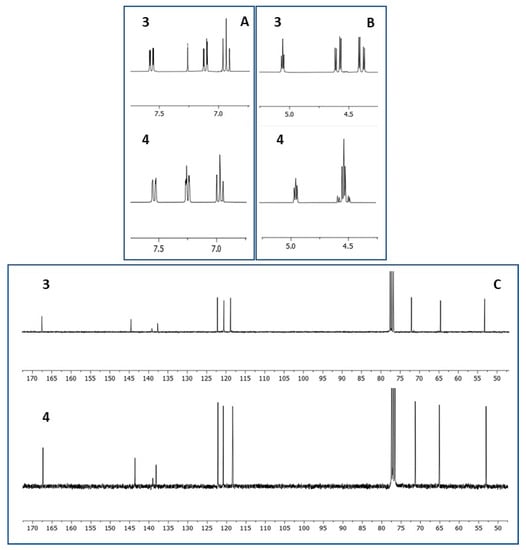
Figure 3.
Aromatic region (A) and aliphatic region (B) of the 1H NMR spectra of 3 and 4 and 13C NMR spectra of 3 and 4 (C). (A) The three adjacent hydrogens of the benzene ring give an analogous sequence of signals: dd, dd and t (from low field to high field). (B) The three hydrogens of the dioxane portion give an analogous sequence of a pseudo triplet and two double doublets or three double doublets. (C) The spectra are indistinguishable. All the spectra were registered in CDCl3.
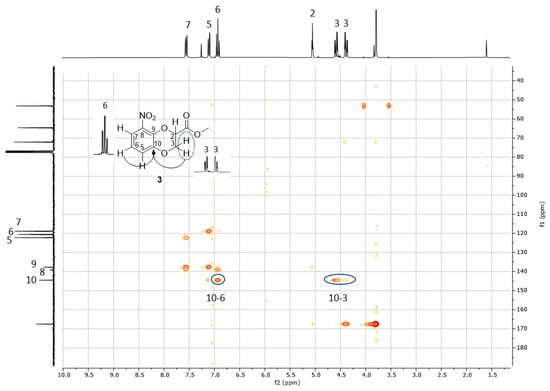
Figure 4.
HMBC spectrum of 3 in CDCl3 displaying important correlations between C(10) and H(6) and between C(10) and H(3).
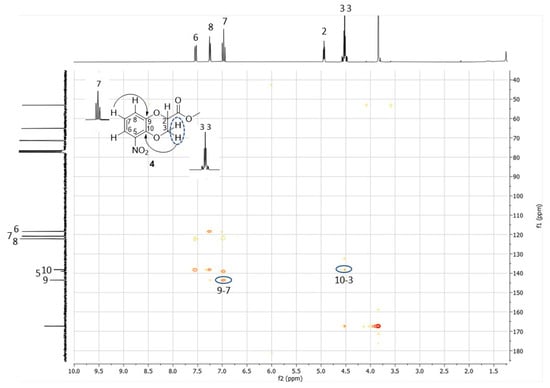
Figure 5.
HMBC spectrum of 4 displaying important correlations between C(9) and H(7) and between C(10) and H(3).
3. Materials and Methods
3-Nitrocatechol was prepared from catechol as previously reported [11]. Methyl 2,3-dibromopropionate was purchased from Sigma-Aldrich S.A. (St. Louis, MO, USA). The 1H and 13C NMR, and two dimensional (2D) (HSQC, and HMBC) spectra were measured on a Varian Mercury 300 FT-NMR spectrometer operating at 300 MHz for 1H and 75 MHz for 13C. NMR data were recorded in CDCl3 at 25 °C, with chemical shifts δ reported in parts per million and coupling constants J in Hertz.
Methyl 8-Nitro-1,4-benzodioxane-2-carboxylate (3) and Methyl-5-nitro-1,4-benzodioxane-2-carboxylate (4)
3-Nitrocatechol (2 g, 12.89 mmol) was dissolved into a stirred solution of N,N-diisopropylethylamine (6.7 mL, 38.68 mmol) in acetonitrile (35 mL). A solution of methyl 2,3-dibromopropionate (3.17 g, 12.89 mmol) in acetonitrile was added dropwise. The mixture was stirred at reflux overnight and, upon cooling to room temperature, diluted with ethyl acetate, washed with water, then with 1 M aqueous NaOH and, finally, with brine. The organic layer was separated, dried with sodium sulphate and concentrated to give the crude mixture of 3 and 4. Diethyl ether (7 mL) was added, and the resulting suspension was vigorously stirred for 1 h at room temperature. Upon filtration, 3 was obtained as a white solid (0.47 g, 15.2%): m.p. 90.2 °C, Rf = 0.45 (8/2 diisopropyl ether/petroleum ether); 1H NMR (300 MHz, CDCl3) δ 7.56 (dd, J = 8.2, 1.6 Hz, 1H), 7.11 (dd, J = 8.2, 1.6 Hz, 1H), 6.93 (t, J = 8.2 Hz, 1H), 5.06 (pseudo t, J = 3.0 Hz, 1H), 4.59 (dd, J = 11.7, 3.0 Hz, 1H), 4.39 (dd, J = 11.7, 3.0 Hz, 1H), 3.80 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 167.5, 144.5, 139.1, 137.7, 122.2, 120.5, 118.8, 72.1, 64.6, 53.2. The filtrate was concentrated, and the resultant crude was purified by flash chromatography on silica gel (gradient elution: from 100% petroleum ether to 80/20 petroleum ether/ethyl acetate) obtaining, in order, 3 (0.49 g, 15.9%) and 4 (0.62 g, 20.1%), which was isolated as a viscous oil. 4: Rf = 0.37 (8/2 diisopropyl ether/petroleum ether); 1H NMR (300 MHz, CDCl3) δ 7.54 (dd, J = 8.2, 1.6 Hz, 1H), 7.25 (dd, J = 8.2, 1.6 Hz, 1H), 6.97 (t, J = 8.2 Hz, 1H), 4.94 (dd, J = 4.4, 3.2 Hz, 1H), 4.59–4.44 (m, 2H), 3.84 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 167.5, 143.7, 139.2, 138.3, 122.4, 121.0, 118.6, 71.5, 65.3, 53.2.
4. Conclusions
In summary, we have obtained the two regioisomeric methyl 1,4-benzodioxane-2-carboxylates nitrated at C(8) (3) and C(5) (4) by reaction of methyl 2,3-dibromopropionate with 3-nitrocatechol. The complete separation of the two isomers was accomplished by precipitation of pure solid 3 from diethyl ether, followed by chromatography of the filtrate to give, in order, residual 3 and pure 4, as an oil. The unambiguous identification of their structures was achieved by HMBC NMR, as recently reported for the 8-bromo and 5-bromo analogues. Straightforwardness and easy product separation connote this synthesis, for which, to our knowledge, alternative approaches have not been reported to date.
Supplementary Materials
The following supporting information can be downloaded online: 1H NMR spectra of 3 and 4 in CDCl3; 13C NMR spectra of 3 and 4 in CDCl3; HSQC spectra of 3 and 4 in CDCl3; HMBC spectra of 3 and 4 in CDCl3.
Author Contributions
Conceptualization, C.B.; methodology, E.A.; investigation, E.A., A.G. and C.M.; writing—review and editing, M.P.; supervision, C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data for the compounds presented in this study are available in the Supplementary Materials of this article.
Acknowledgments
The authors acknowledge support from the University of Milan through the APC initiative.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bolchi, C.; Bavo, F.; Appiani, R.; Roda, G.; Pallavicini, M. 1,4-Benzodioxane, an evergreen, versatile scaffold in medicinal chemistry: A review of its recent applications in drug design. Eur. J. Med. Chem. 2020, 200, 112419. [Google Scholar] [CrossRef] [PubMed]
- Muszak, D.; Surmiak, E.; Plewka, J.; Magiera-Mularz, K.; Kocik-Krol, J.; Musielak, B.; Sala, D.; Kitel, R.; Stec, M.; Weglarczyk, K.; et al. Terphenyl-based small-molecule inhibitors of programmed cell death-1/programmed death-ligand 1 protein-protein interaction. J. Med. Chem. 2021, 64, 11614–11636. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Vibhute, S.; Li, L.; Okumu, A.; Ratigan, S.C.; Nolan, S.; Papa, J.L.; Mann, C.A.; English, A.; Chen, A.; et al. Optimization of TopoIV potency, ADMET properties, and hERG inhibition of 5-amino-1,3-dioxane-linked novel bacterial topoisomerase inhibitors: Identification of a lead with in vivo efficacy against MRSA. J. Med. Chem. 2021, 64, 15214–15249. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, A.E.; Sharp, S.Y.; Chessum, N.E.A.; Hayes, A.; Pellegrino, L.; Tucker, M.J.; Miah, A.; Wilding, B.; Evans, L.E.; Rye, C.S.; et al. HSF1pahway inhibitor clinical candidate (CCT361814/NXP800) developed from a phenotypic screen as a potential treatment for refractory ovarian cancer and other malignancies. J. Med. Chem. 2023, 66, 5907–5936. [Google Scholar] [CrossRef] [PubMed]
- Bolchi, C.; Valoti, E.; Straniero, V.; Ruggeri, P.; Pallavicini, M. From 2-aminomethyl-1,4-benzodioxane enantiomers to unichiral 2-cyano- and 2-carbonyl-substituted benzodioxanes via dichloroamine. J. Org. Chem. 2014, 79, 6732–6737. [Google Scholar] [CrossRef] [PubMed]
- Bavo, F.; Pallavicini, M.; Gotti, C.; Appiani, R.; Moretti, M.; Colombo, S.F.; Pucci, S.; Viani, P.; Budriesi, R.; Renzi, M.; et al. Modifications at C(5) of 2-(2-pyrrolidinyl)-substituted 1,4-benzodioxane elicit potent α4β2 nicotinic acetylcholine receptor partial agonism with high selectivity over the α3β4 subtype. J. Med. Chem. 2020, 63, 15668–15692. [Google Scholar] [CrossRef] [PubMed]
- Bavo, F.; Pallavicini, M.; Appiani, R.; Bolchi, C. Determinants for α4β2 vs. α3β4 subtype selectivity of pyrrolidine-based nAChRs ligands: A computational perspective with focus on recent cryo-em receptor structures. Molecules 2021, 26, 3603. [Google Scholar] [CrossRef] [PubMed]
- Bouissane, L.; Khouili, M.; Coudert, G.; Pujol, M.D.; Guillaumet, G. New and promising type of leukotriene B4 (LTB4) antagonists based on the 1,4-benzodioxine structure. Eur. J. Med. Chem. 2023, 254, 115332. [Google Scholar] [CrossRef] [PubMed]
- Bolchi, C.; Bavo, F.; Pallavicini, M. Preparation and unequivocal identification of the regioisomers of nitrocatechol monobenzyl ether. Synth. Commun. 2017, 47, 1507–1513. [Google Scholar] [CrossRef]
- Armano, E.; Giraudo, A.; Pallavicini, M.; Bolchi, C. Methyl 8- and 5-bromo-1,4-benzodioxane-2-carboxylate: Unambiguous identification of the two regioisomers. Molbank 2023, 2023, M1623. [Google Scholar] [CrossRef]
- Elgaher, W.A.M.; Hamed, M.M.; Baumann, S.; Herrmann, J.; Siebenbürger, L.; Krull, J.; Cirnski, K.; Kirschning, A.; Brönstrup, M.; Müller, R.; et al. Cystobactamid 507: Concise synthesis, mode of action, and optimization toward more potent antibiotics. Chem. Eur. J. 2020, 26, 7219–7225. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).