[4,6-Di-tert-butyl-N-(2,6-Dimethylphenyl)-o-Amidophenolato][4,6-Di-tert-butyl-N-(2,6-Dimethylphenyl)-o-Iminobenzosemiquinolato](2,2′-Bipyridyl)Indium(III)
Abstract
1. Introduction
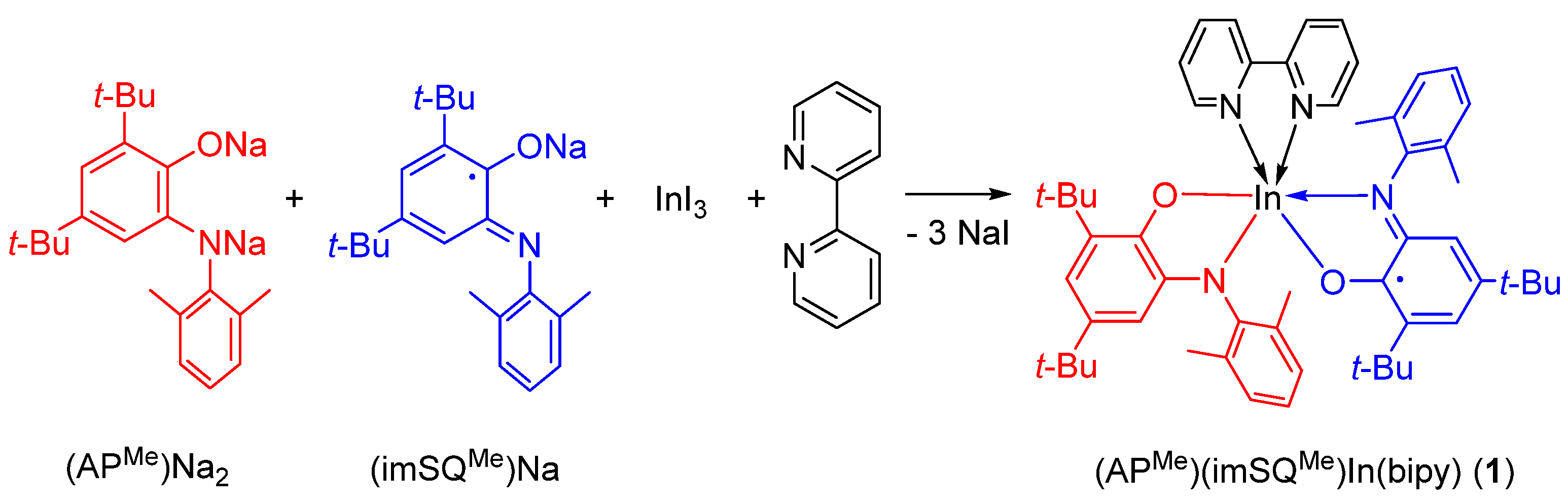
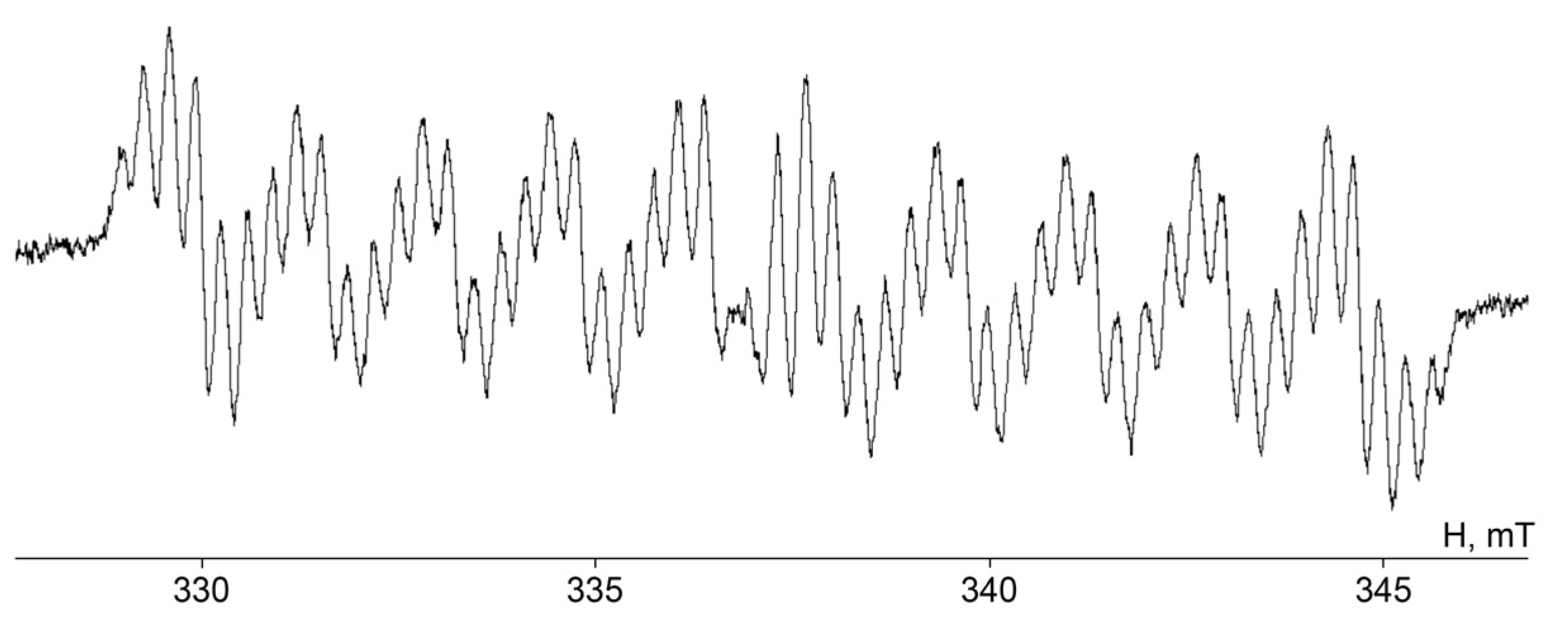
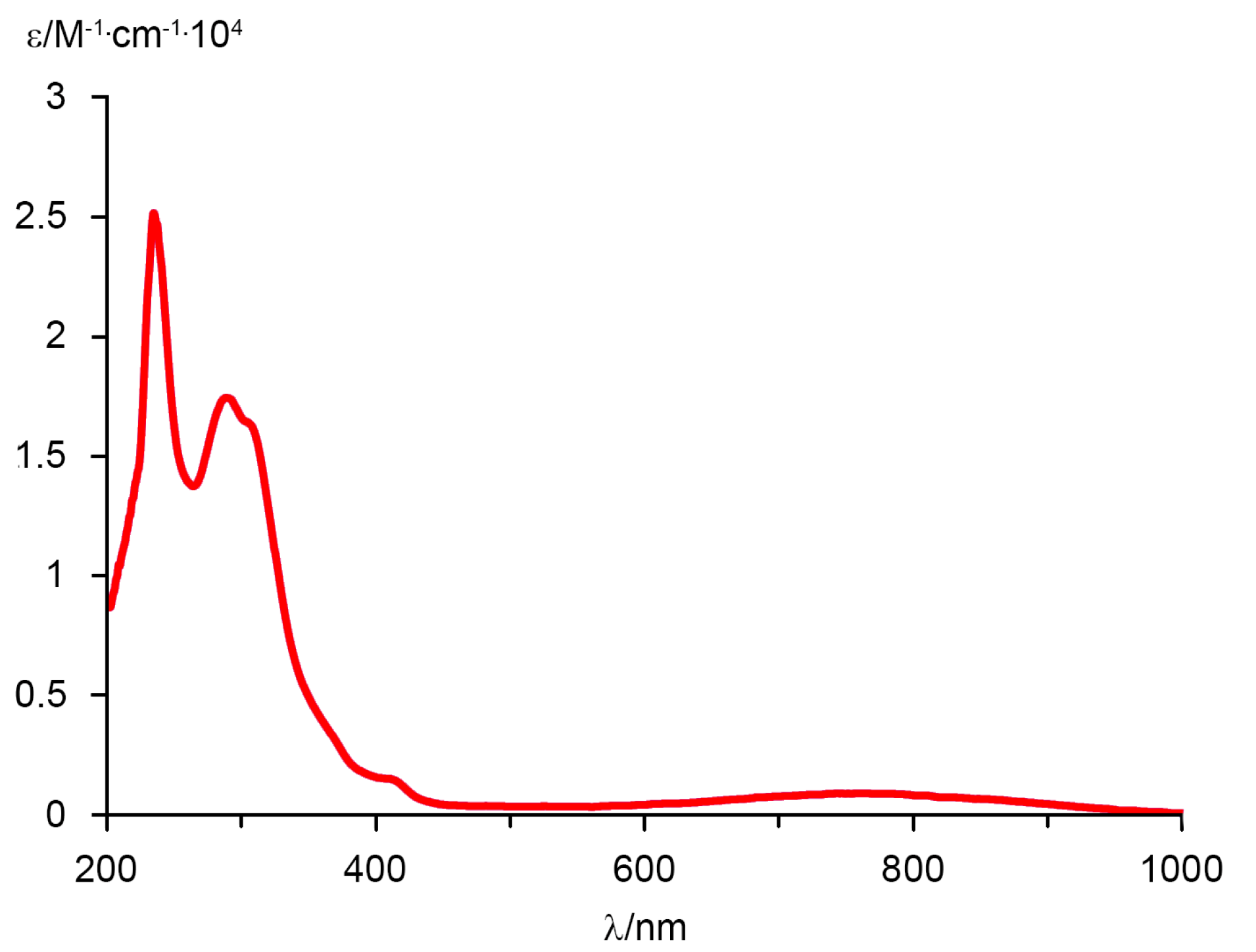
2. Results
3. Materials and Methods
3.1. Synthesis of 1
3.2. Single-Crystal X-ray Structure Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, G.; Wang, Z.Y. Near-Infrared Organic Compounds and Emerging Applications. Chem. Asian J. 2010, 5, 1006–1029. [Google Scholar] [CrossRef]
- Gerasimova, T.P.; Shamsieva, A.V.; Strel’nik, I.D.; Katsyuba, S.A.; Musina, E.I.; Karasik, A.A.; Sinyashin, O.G. Study of the structures and photophysical properties of 1,3-diaza-5-phosphacyclohexanes using density functional theory and optical spectroscopy. Russ. Chem. Bull. 2020, 69, 449–457. [Google Scholar] [CrossRef]
- Kalinin, A.A.; Sharipova, S.M.; Islamova, L.N.; Fazleeva, G.M.; Busyurova, D.N.; Sharipova, A.V.; Fominykh, O.D.; Balakina, M.Y. Chromophores with quinoxaline core in π-bridge and aniline or carbazole donor moiety: Synthesis and comparison of their linear and nonlinear optical properties. Russ. Chem. Bull. 2022, 71, 1009–1018. [Google Scholar] [CrossRef]
- Wu, J.; Shi, Z.; Zhu, L.; Li, J.; Han, X.; Xu, M.; Hao, S.; Fan, Y.; Shao, T.; Bai, H.; et al. The Design and Bioimaging Applications of NIR Fluorescent Organic Dyes with High Brightness. Adv. Opt. Mater. 2022, 10, 2102514. [Google Scholar] [CrossRef]
- Marechal, E. Polymeric dyes—Synthesis, properties and uses. Prog. Org. Coat. 1982, 10, 251–287. [Google Scholar] [CrossRef]
- Peng, D.; Tang, G.; Hu, J.; Xie, Q.; Zhou, J.; Zhang, W.; Zhong, C. Novel D–π–A dye sensitizers of polymeric metal complexes with triphenylamine or carbazole derivatives as donor for dye-sensitized solar cells: Synthesis, characterization and application. Polym. Bull. 2015, 72, 653–669. [Google Scholar] [CrossRef]
- Ho, C.-L.; Li, H.; Wong, W.-Y. Red to near-infrared organometallic phosphorescent dyes for OLED applications. J. Organomet. Chem. 2014, 751, 261–285. [Google Scholar] [CrossRef]
- Saygili, Y.; Stojanovic, M.; Flores-Díaz, N.; Zakeeruddin, S.M.; Vlachopoulos, N.; Grätzel, M.; Hagfeldt, A. Metal coordination complexes as redox mediators in regenerative dye-sensitized solar cells. Inorganics 2019, 7, 30. [Google Scholar] [CrossRef]
- Kim, D.; Gra, T.G.; Teets, T.S. Heteroleptic copper(i) charge-transfer chromophores with panchromatic absorption. Chem. Commun. 2022, 58, 11446–11449. [Google Scholar] [CrossRef]
- Pashanova, K.I.; Ershova, I.V.; Trofimova, O.Y.; Rumyantsev, R.V.; Fukin, G.K.; Bogomyakov, A.S.; Arsenyev, M.V.; Piskunov, A.V. Charge Transfer Chromophores Derived from 3d-Row Transition Metal Complexes. Molecules 2022, 27, 8175. [Google Scholar] [CrossRef]
- Li, G.; Jiang, Z.; Tang, M.; Jiang, X.; Tu, H.; Zhu, S.; Liu, R.; Zhu, H. Synthesis, Photophysics and Tunable Reverse Saturable Absorption of Bis-Tridentate Iridium(III) Complexes via Modification on Diimine Ligand. Molecules 2023, 28, 566. [Google Scholar] [CrossRef]
- Shultz, D.A.; Stephenson, R.; Kirk, M.L. Dinuclear ligand-to-ligand charge transfer complexes. Dalton Trans. 2023, 52, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Younus, M.; Valandro, S.; Gobeze, H.B.; Ahmed, S.; Schanze, K.S. Wavelength and solvent controlled energy and charge transfer in donor-acceptor substituted platinum acetylide complexes. J. Photochem. Photobiol. A Chem. 2023, 435, 114303. [Google Scholar] [CrossRef]
- Cummings, S.D.; Cheng, L.-T.; Eisenberg, R. Metalloorganic Compounds for Nonlinear Optics: Molecular Hyperpolarizabilities of M(diimine)(dithiolate) Complexes (M = Pt, Pd, Ni). Chem. Mater. 1997, 9, 440–450. [Google Scholar] [CrossRef]
- Base, K.; Tierney, M.T.; Fort, A.; Muller, J.; Grinstaff, M.W. On the Second-Order Nonlinear Optical Structure−Property Relationships of Metal Chromophores. Inorg. Chem. 1999, 38, 287–289. [Google Scholar] [CrossRef]
- Bonneval, B.G.-d.; Ching, K.I.M.-C.; Alary, F.; Bui, T.-T.; Valade, L. Neutral d8 metal bis-dithiolene complexes: Synthesis, electronic properties and applications. Coord. Chem. Rev. 2010, 254, 1457–1467. [Google Scholar] [CrossRef]
- Deplano, P.; Pilia, L.; Espa, D.; Mercuri, M.L.; Serpe, A. Square-planar d8 metal mixed-ligand dithiolene complexes as second order nonlinear optical chromophores: Structure/property relationship. Coord. Chem. Rev. 2010, 254, 1434–1447. [Google Scholar] [CrossRef]
- Archer, S.; Weinstein, J.A. Charge-separated excited states in platinum(II) chromophores: Photophysics, formation, stabilization and utilization in solar energy conversion. Coord. Chem. Rev. 2012, 256, 2530–2561. [Google Scholar] [CrossRef]
- Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E. Light harvesting with Earth abundant d-block metals: Development of sensitizers in dye-sensitized solar cells (DSCs). Coord. Chem. Rev. 2013, 257, 3089–3106. [Google Scholar] [CrossRef]
- Scattergood, P.A.; Jesus, P.; Adams, H.; Delor, M.; Sazanovich, I.V.; Burrows, H.D.; Serpa, C.; Weinstein, J.A. Exploring excited states of Pt(II) diimine catecholates for photoinduced charge separation. Dalton Trans. 2015, 44, 11705–11716. [Google Scholar] [CrossRef]
- Kramer, W.W.; Cameron, L.A.; Zarkesh, R.A.; Ziller, J.W.; Heyduk, A.F. Donor–Acceptor Ligand-to-Ligand Charge-Transfer Coordination Complexes of Nickel(II). Inorg. Chem. 2014, 53, 8825–8837. [Google Scholar] [CrossRef]
- Bubnov, M.P.; Teplova, I.A.; Druzhkov, N.O.; Fukin, G.K.; Cherkasova, A.V.; Cherkasov, V.K. Catecholato complexes of cobalt and nickel with 1,4-disubstituted-1,4-diazabutadiens-1,3 and 1,2-bis(diphenylphosphino)ethane. J. Chem. Sci. 2015, 127, 527–535. [Google Scholar] [CrossRef]
- Cameron, L.A.; Ziller, J.W.; Heyduk, A.F. Near-IR absorbing donor–acceptor ligand-to-ligand charge-transfer complexes of nickel(II). Chem. Sci. 2016, 7, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, Y.; Liu, B.; Su, J.-H.; Fedushkin, I.L.; Wua, B.; Yang, X.-J. Noninnocent ligands: Heteroleptic nickel complexes with α-diimine and 1,2-diketone derivatives. Dalton Trans. 2017, 46, 7857–7865. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Matsumoto, T.; Chang, H.-C. Impact of Group 10 Metals on the Solvent-Induced Disproportionation of o-Semiquinonato Complexes. Chem. Eur. J. 2019, 25, 8268–8278. [Google Scholar] [CrossRef] [PubMed]
- Pashanova, K.I.; Bitkina, V.O.; Yakushev, I.A.; Arsenyev, M.V.; Piskunov, A.V. Square-Planar Heteroleptic Complexes of α-Diimine-NiII-Catecholate Type: Intramolecular Ligand-to-Ligand Charge Transfer. Molecules 2021, 26, 4622. [Google Scholar] [CrossRef]
- Kokatam, S.-L.; Chaudhuri, P.; Weyhermüller, T.; Wieghardt, K. Molecular and electronic structure of square planar complexes [PdII(tbpy)(LIPN,O)]0, [PdII(tbpy)(LISQN,O)](PF6), and [PdII(tbpy)(LIBQN,O)](PF6)(BF4)·2CH2Cl2: An o-iminophenolato based ligand centered, three-membered redox series. Dalton Trans. 2007, 3, 373–378. [Google Scholar] [CrossRef]
- BaniKhaled, M.O.; Becker, J.D.; Koppang, M.; Sun, H. Perfluoroalkylation of Square-Planar Transition Metal Complexes: A Strategy To Assemble Them into Solid State Materials with a π–π Stacked Lamellar Structure. Cryst. Growth Des. 2016, 16, 1869–1878. [Google Scholar] [CrossRef]
- Tahara, K.; Ashihara, Y.; Higashino, T.; Ozawa, Y.; Kadoya, T.; Sugimoto, K.; Ueda, A.; Morib, H.; Abe, M. New π-extended catecholato complexes of Pt(ii) and Pd(ii) containing a benzothienobenzothiophene (BTBT) moiety: Synthesis, electrochemical behavior and charge transfer properties. Dalton Trans. 2019, 48, 7367–7377. [Google Scholar] [CrossRef]
- Heinze, K.; Reinhardt, S. Platinum(II) Complexes with Non-Innocent Ligands: Solid-Phase Synthesis, Redox Chemistry and Luminescence. Chem. Eur. J. 2008, 14, 9482–9486. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Davies, E.S.; Adams, H.; Best, J.; Weinstein, J.A. Platinum(II) Diimine Complexes with Catecholate Ligands Bearing Imide Electron-Acceptor Groups: Synthesis, Crystal Structures, (Spectro)Electrochemical and EPR studies, and Electronic Structure. Inorg. Chem. 2008, 47, 1532–1547. [Google Scholar] [CrossRef] [PubMed]
- Best, J.; Sazanovich, I.V.; Adams, H.; Bennett, R.D.; Davies, E.S.; Meijer, A.J.H.M.; Towrie, M.; Tikhomirov, S.A.; Bouganov, O.V.; Ward, M.D.; et al. Structure and Ultrafast Dynamics of the Charge-Transfer Excited State and Redox Activity of the Ground State of Mono- and Binuclear Platinum(II) Diimine Catecholate and Bis-catecholate Complexes: A Transient Absorption, TRIR, DFT, and Electrochemical Study. Inorg. Chem. 2010, 49, 10041–10056. [Google Scholar] [CrossRef]
- Sobottka, S.; Nößler, M.; Ostericher, A.L.; Hermann, G.; Subat, N.Z.; Beerhues, J.; Meer, M.B.-v.d.; Suntrup, L.; Albold, U.; Hohloch, S.; et al. Tuning PtII-Based Donor–Acceptor Systems through Ligand Design: Effects on Frontier Orbitals, Redox Potentials, UV/Vis/NIR Absorptions, Electrochromism, and Photocatalysis. Chem. Eur. J. 2020, 26, 1314–1327. [Google Scholar] [CrossRef]
- Maleeva, A.V.; Ershova, I.V.; Trofimova, O.Y.; Arsenyeva, K.V.; Yakushev, I.A.; Piskunov, A.V. Near-IR absorbing donor-acceptor charge-transfer gallium complex—An example from non-transition metal chemistry. Mendeleev Commun. 2022, 32, 83–86. [Google Scholar] [CrossRef]
- Maleeva, A.V.; Trofimova, O.Y.; Ershova, I.V.; Arsenyeva, K.V.; Pashanova, K.I.; Yakushev, I.A.; Cherkasov, A.V.; Aysin, R.R.; Piskunov, A.V. Molecular and electronic structures of paramagnetic gallium complexes with differently charged o-quinone ligands. Russ. Chem. Bull. 2022, 71, 1441–1452. [Google Scholar] [CrossRef]
- Ershova, I.V.; Maleeva, A.V.; Aysin, R.R.; Cherkasov, A.V.; Piskunov, A.V. Effect of crystal packing on charge transfer in the heteroleptic gallium(III) complex. Russ. Chem. Bull. 2023, 72, 193–201. [Google Scholar] [CrossRef]
- Emsley, J. The Elements; Clarendon Press: Oxford, UK, 1991. [Google Scholar]
- Piskunov, A.V.; Mescheryakova, I.N.; Fukin, G.K.; Cherkasov, V.K.; Abakumov, G.A. Indium(III) complexes with o-iminobenzoquinone in different redox states. New J. Chem. 2010, 34, 1746–1750. [Google Scholar] [CrossRef]
- Piskunov, A.V.; Meshcheryakova, I.N.; Fukin, G.K.; Shavyrin, A.S.; Cherkasov, V.K.; Abakumov, G.A. The new C–C bond formation in the reaction of o-amidophenolate indium(III) complex with alkyl iodides. Dalton Trans. 2013, 42, 10533–10539. [Google Scholar] [CrossRef]
- Piskunov, A.V.; Meshcheryakova, I.N.; Ershova, I.V.; Bogomyakov, A.S.; Cherkasov, A.V.; Fukin, G.K. The reactivity of o-amidophenolate indium(III) complexes towards different oxidants. RSC Adv. 2014, 4, 42494–42505. [Google Scholar] [CrossRef]
- Piskunov, A.V.; Maleeva, A.V.; Fukin, G.K.; Baranov, E.V.; Bogomyakov, A.S.; Cherkasov, V.K.; Abakumov, G.A. Synthesis and molecular structure of indium complexes based on 3,6-di-tert-butyl-o-benzoquinone. Looking for indium(I) o-semiquinolate. Dalton Trans. 2011, 40, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.N. Metrical Oxidation States of 2-Amidophenoxide and Catecholate Ligands: Structural Signatures of Metal−Ligand π Bonding in Potentially Noninnocent Ligands. Inorg. Chem. 2012, 51, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D.D.; Armarego, W.L.F.; Perrin, D.R. Purification of Laboratory Chemicals; Pergamon Press: Oxford, UK, 1980. [Google Scholar]
- Abakumov, G.A.; Druzhkov, N.O.; Kurskii, Y.A.; Shavyrin, A.S. NMR study of products of thermal transformation of substituted N-aryl-o-quinoneimines. Russ. Chem. Bull. Int. Ed. 2003, 52, 712–717. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction, CrysAlisPro Software System, ver. 1.171.42.72a. Rigaku Corpo-ration: Wroclaw, Poland, 2022.
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ershova, I.V.; Cherkasov, A.V.; Piskunov, A.V. [4,6-Di-tert-butyl-N-(2,6-Dimethylphenyl)-o-Amidophenolato][4,6-Di-tert-butyl-N-(2,6-Dimethylphenyl)-o-Iminobenzosemiquinolato](2,2′-Bipyridyl)Indium(III). Molbank 2023, 2023, M1660. https://doi.org/10.3390/M1660
Ershova IV, Cherkasov AV, Piskunov AV. [4,6-Di-tert-butyl-N-(2,6-Dimethylphenyl)-o-Amidophenolato][4,6-Di-tert-butyl-N-(2,6-Dimethylphenyl)-o-Iminobenzosemiquinolato](2,2′-Bipyridyl)Indium(III). Molbank. 2023; 2023(2):M1660. https://doi.org/10.3390/M1660
Chicago/Turabian StyleErshova, Irina V., Anton V. Cherkasov, and Alexandr V. Piskunov. 2023. "[4,6-Di-tert-butyl-N-(2,6-Dimethylphenyl)-o-Amidophenolato][4,6-Di-tert-butyl-N-(2,6-Dimethylphenyl)-o-Iminobenzosemiquinolato](2,2′-Bipyridyl)Indium(III)" Molbank 2023, no. 2: M1660. https://doi.org/10.3390/M1660
APA StyleErshova, I. V., Cherkasov, A. V., & Piskunov, A. V. (2023). [4,6-Di-tert-butyl-N-(2,6-Dimethylphenyl)-o-Amidophenolato][4,6-Di-tert-butyl-N-(2,6-Dimethylphenyl)-o-Iminobenzosemiquinolato](2,2′-Bipyridyl)Indium(III). Molbank, 2023(2), M1660. https://doi.org/10.3390/M1660





