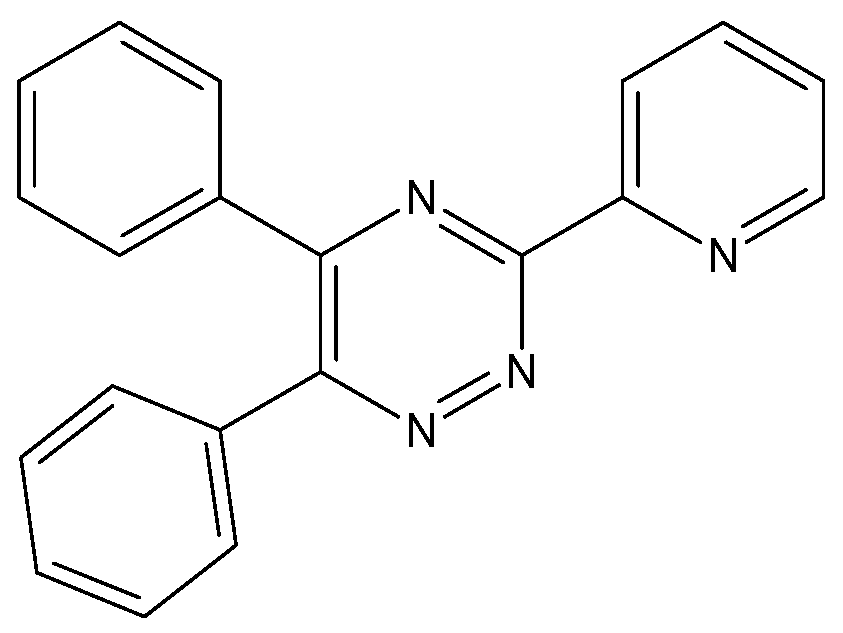
2-(5,6-Diphenyl-1,2,4-Triazin-3-yl)pyridinium Dichloroiodate (I)
Abstract
1. Introduction
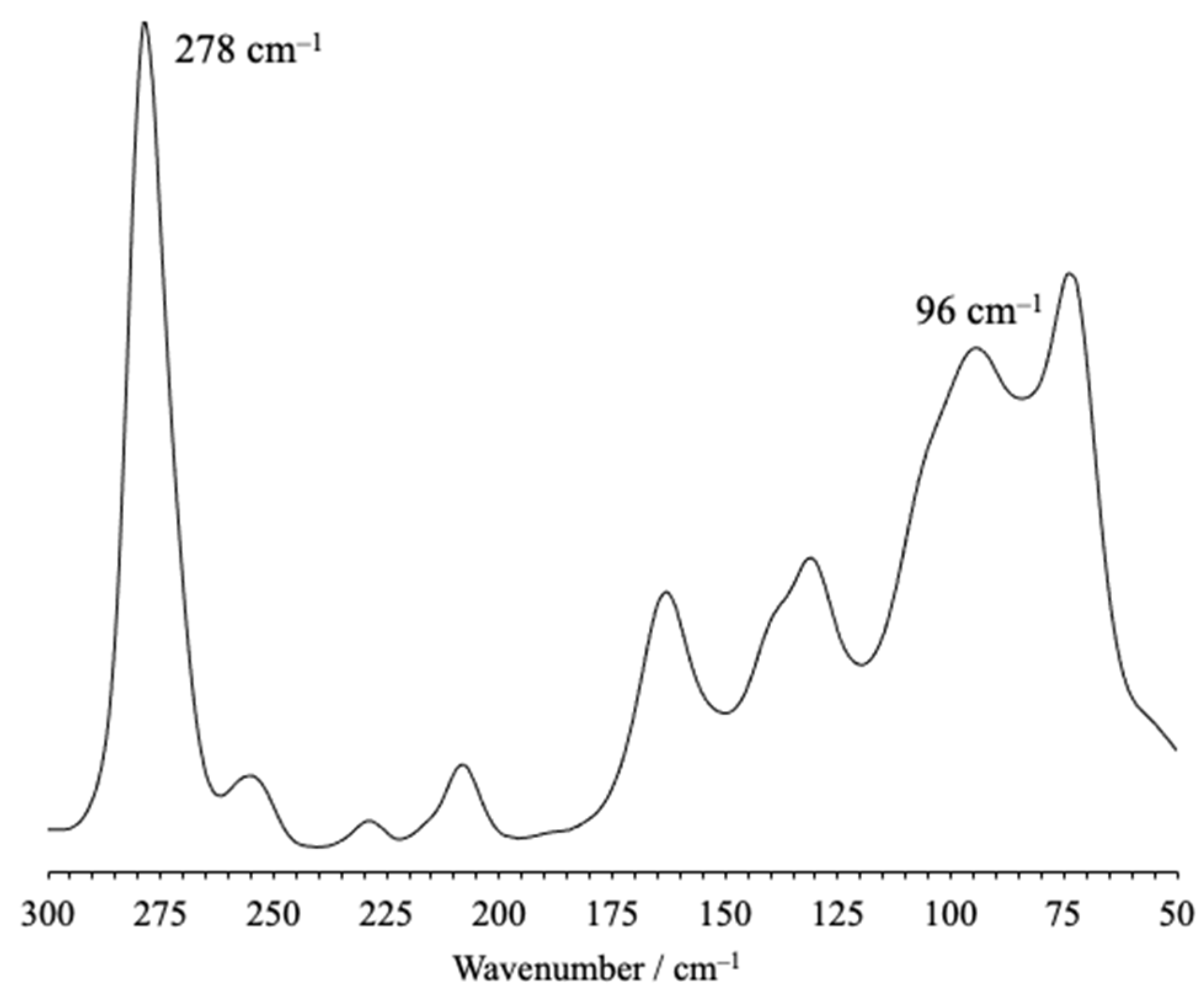
2. Results
3. Materials and Methods
3.1. General
3.2. Synthesis of 2-(5,6-Diphenyl-1,2,4-Triazin-3-yl)pyridinium Dichloroiodate (I) (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rimmer, E.L.; Bailey, R.D.; Pennington, W.T.; Hanks, T.W. The reaction of iodine with 9-methylacridine: Formation of polyiodide salts and a charge-transfer complex. J. Chem. Soc. Perkin Trans. 2 1998, 11, 2557–2562. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Podda, E.; Arca, M.; Pintus, A.; Lippolis, V.; Caltagirone, C.; Bartz, R.H.; Lenardão, E.J.; Perin, G.; Schumacher, R.F.; et al. An unprecedented non-classical polyinterhalogen anion made of [I2Cl]− and I2 at the 2-(p-tolyl)selenopheno [2,3-b]pyridinium cation template. N. J. Chem. 2022, 46, 21921–21929. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Devillanova, F.A.; Hursthouse, M.B.; Huth, S.L.; Isaia, F.; Lippolis, V.; Mancini, A.; Ogilvie, H.R.; Verani, G. Reactions of pyridyl donors with halogens and interhalogens: An X-ray diffraction and FT-Raman investigation. J. Organomet. Chem. 2005, 690, 1923–1934. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Devillanova, F.A.; Hursthouse, M.B.; Huth, S.L.; Isaia, F.; Lippolis, V.; Mancini, A.; Verani, G. Reactions of Halogens/Interhalogens with Polypyridyl Substrates: The Case of 2,4,6-Tris(2-pyridyl)-1,3,5-triazine. Eur. J. Inorg. Chem. 2008, 3921–3928. [Google Scholar] [CrossRef]
- Kukkonen, E.; Malinen, H.; Haukka, M.; Konu, J. Reactivity of 4-Aminopyridine with Halogens and Interhalogens: Weak Interactions Supported Networks of 4-Aminopyridine and 4-Aminopyridinium. Cryst. Growth Des. 2019, 19, 2434–2445. [Google Scholar] [CrossRef]
- Tuikka, M.; Haukka, M. Crystal structure of the pyridine–diiodine (1/1) adduct. Acta Cryst. 2015, e71, o463. [Google Scholar] [CrossRef]
- Batsanov, A.S.; Lightfoot, A.P.; Twiddle, S.J.R.; Whiting, A. Stereoselective Chloro-Deboronation Reactions Induced by Substituted Pyridine–Iodine Chloride Complexes. Eur. J. Org. Chem. 2005, 1876–1883. [Google Scholar] [CrossRef]
- Batsanov, A.S.; Lightfoot, A.P.; Twiddle, S.J.R.; Whiting, A. Bis(2,6-dimethylpyridyl)iodonium dibromoiodate. Acta Cryst. 2006, e62, 0901–0902. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Devillanova, F.A.; Hursthouse, M.B.; Huth, S.L.; Isaia, F.; Lippolis, V.; Mancini, A. Square-pyramidal bonding of I2 molecules at the I− nodes of a polyiodide infinite pseudo-cubic 3D-network. CrystEngComm 2004, 6, 540–542. [Google Scholar] [CrossRef]
- Pandeeswaran, M.; Elango, K.P. Electronic, Raman and FT-IR spectral investigations of the charge transfer interactions between ketoconazole and povidone drugs with iodine. Spectrochim. Acta A 2009, 72, 789–795. [Google Scholar] [CrossRef]
- Eltayeb, N.E.; Teoh, S.G.; Ng, S.-L.; Fun, H.-K.; Ibrahim, K. 5,6-Diphenyl-3-(2-pyridyl)-1,2,4-triazine. Acta Crystallogr. 2007, e63, o1041–o1042. [Google Scholar] [CrossRef]
- Eltayeb, N.E.; Teoh, S.G.; Chantrapromma, S.; Fun, H.-K.; Ibrahim, K. A second monoclinic polymorph of 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine. Acta Crystallogr. 2007, e63, o3792–o3793. [Google Scholar] [CrossRef]
- Uma, R.; Palaniandavar, M.; Butcher, R.J. Synthesis, structure, spectra and redox interconversions in copper(II) complexes of 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine. J. Chem. Soc. Dalton Trans. 1996, 2061–2066. [Google Scholar] [CrossRef]
- Therrien, B.; Säid-Mohamed, C.; Süss-Fink, G. Mononuclear arene ruthenium complexes containing 5,6-diphenyl-3-(pyridine-2-yl)-1,2,4-triazine as chelating ligand: Synthesis and molecular structure. Inorg. Chim. Acta 2008, 361, 2601–2608. [Google Scholar] [CrossRef]
- Anjomshoa, M.; Hadadzadeh, H.; Torkzadeh-Mahani, M.; Fatemi, S.J.; Adeli-Sardou, M.; Amiri Rudbari, H.; Mollica Nardo, V. A mononuclear Cu(II) complex with 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine: Synthesis, crystal structure, DNA- and BSA-binding, molecular modeling, and anticancer activity against MCF-7, A-549, and HT-29 cell lines. Eur. J. Med. Chem. 2015, 96, 66–82. [Google Scholar] [CrossRef]
- Pathaw, L.; Khamrang, T.; Kathiravan, A.; Velusamy, M. Synthesis, crystal structure, bovine serum albumin binding studies of 1,2,4-triazine based copper(I) complexes. J. Mol. Struct. 2020, 1207, 127821. [Google Scholar] [CrossRef]
- Sonnenberg, K.; Mann, L.; Redeker, F.A.; Schmidt, B.; Riedel, S. Polyhalogen and Polyinterhalogen Anions from Fluorine to Iodine. Angew. Chem. Int. Ed. Engl. 2020, 59, 5464–5493. [Google Scholar] [CrossRef]
- Hallen, H.; Riedel, S. Recent Discoveries of Polyhalogen Anions—From Bromine to Fluorine. Z. Anorg. Allg. Chem. 2014, 640, 1281–1291. [Google Scholar] [CrossRef]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory; Wiley-VCH: New York, NY, USA, 2001; ISBN 978-3-527-30372-4. [Google Scholar]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Tan, K.H.; Taylor, M.J. Vibrational spectra of adducts of iodine monochloride with some pyridine bases. Aust. J. Chem. 1980, 33, 1743–1751. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Re-finement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 (Rev. B01); Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models. J. Chem. Phys. 1998, 108, 664–675. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. (Eds.) GaussView, Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016.
- Mancini, A.; Aragoni, M.C.; Bingham, A.L.; Castellano, C.; Coles (née Huth), S.L.; Demartin, F.; Hursthouse, M.B.; Isaia, F.; Lippolis, V.; Maninchedda, G.; et al. Reactivity of Fluoro-Substituted Bis(thiocarbonyl) Donors with Diiodine: An XRD, FT–Raman, and DFT Investigation. Chem.—Asian J. 2013, 8, 3071–3078. [Google Scholar] [CrossRef]
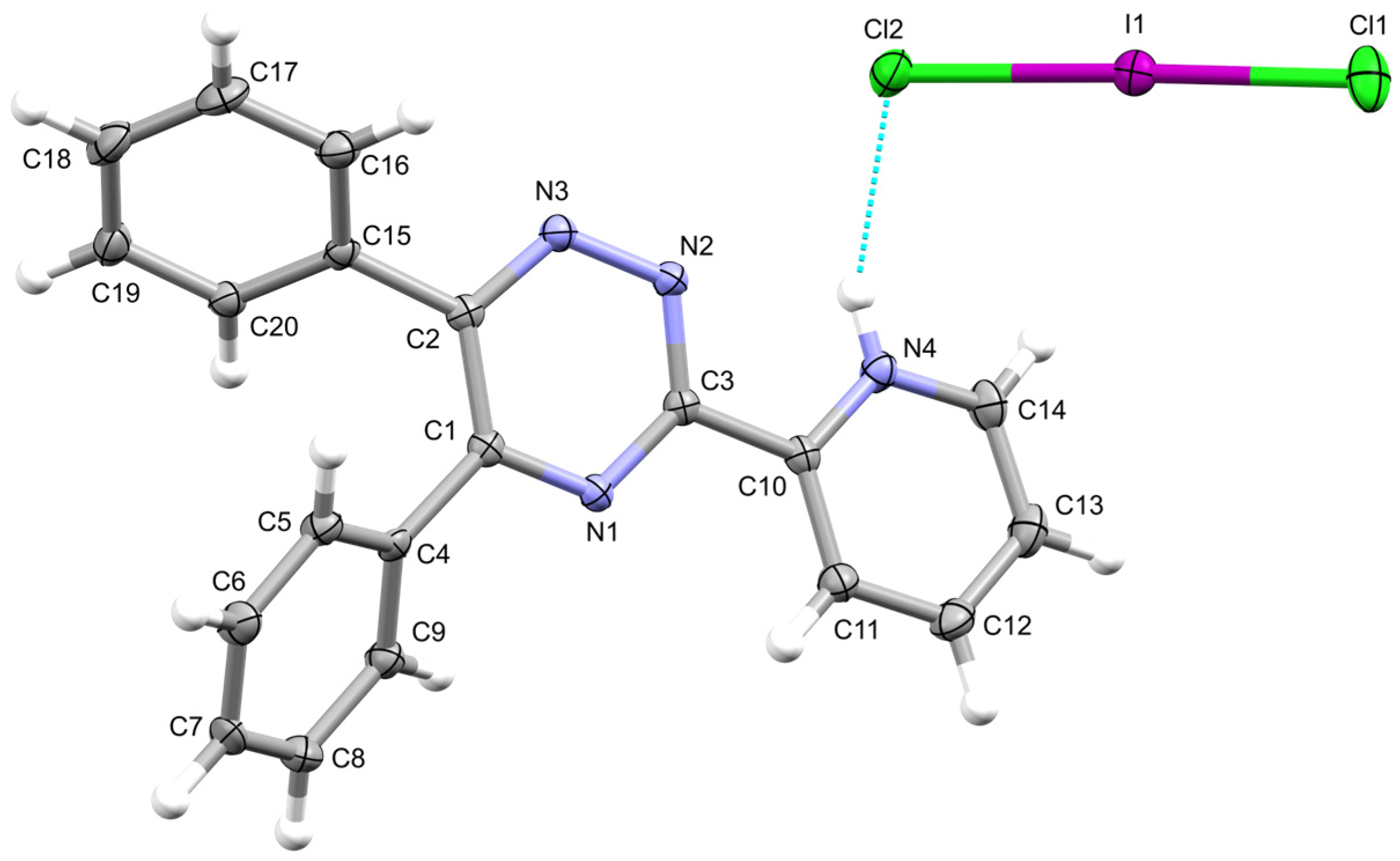
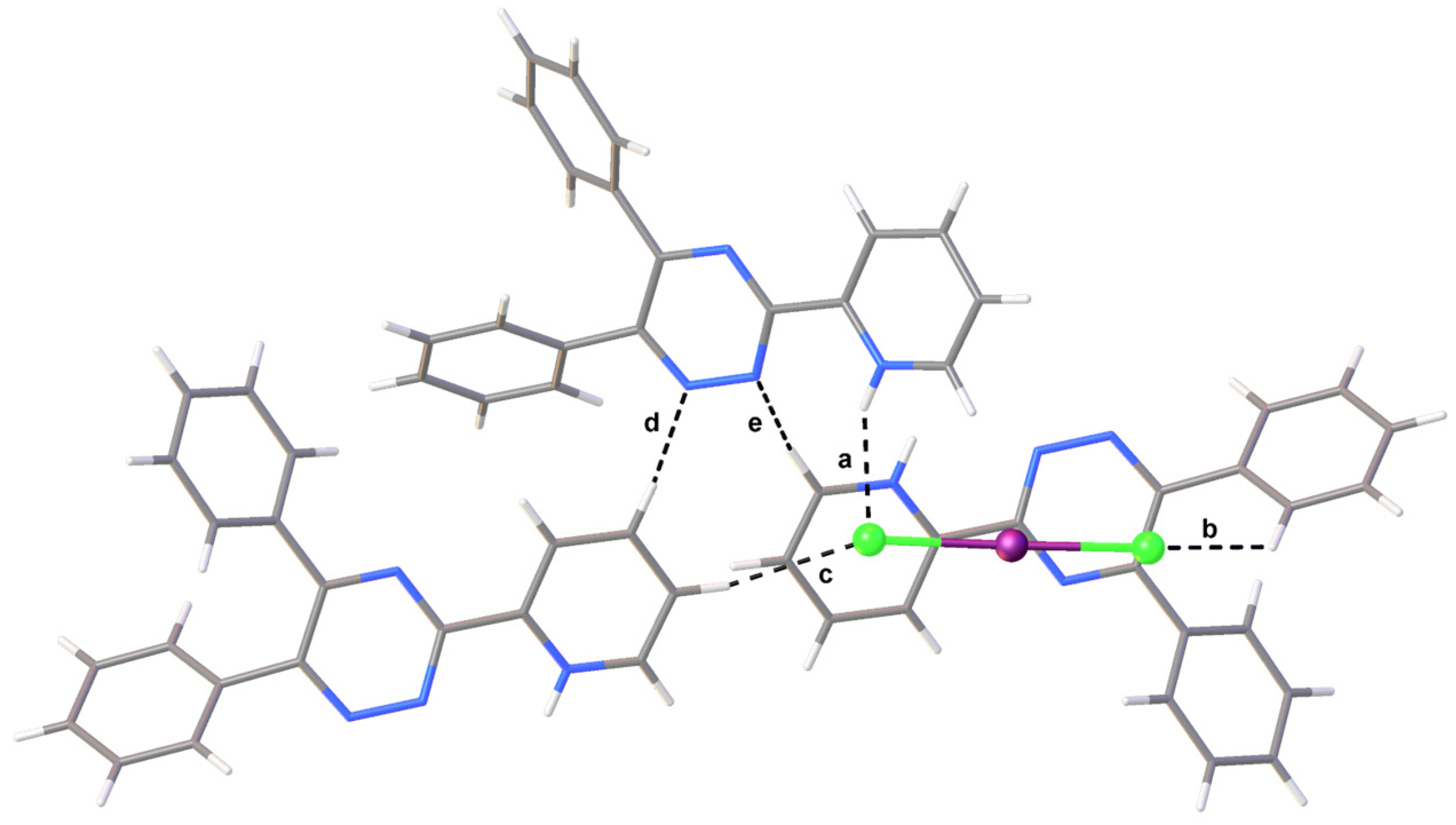
| Interaction | dD–H (Å) | dH∙∙∙A (Å) | dD∙∙∙A (Å) | αD–H···A (°) | |
|---|---|---|---|---|---|
| a | N4–H4∙∙∙Cl2 | 0.85(3) | 2.39(2) | 3.174(2) | 153(2) |
| b | C20i–H20i∙∙∙Cl1 | 0.95 | 2.88 | 3.541(2) | 128.2 |
| c | C13ii–H13ii∙∙∙Cl2 | 0.95 | 2.70 | 3.610(3) | 160.9 |
| d | C12ii –H12ii ∙∙∙N3 | 0.95 | 2.66 | 3.291(3) | 124.5 |
| e | C14i–H14i···N2 | 0.95 | 2.62 | 3.425(3) | 143.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragoni, M.C.; Lippolis, V.; Mancini, A.; Pintus, A.; Podda, E.; Orton, J.B.; Coles, S.J.; Arca, M. 2-(5,6-Diphenyl-1,2,4-Triazin-3-yl)pyridinium Dichloroiodate (I). Molbank 2023, 2023, M1662. https://doi.org/10.3390/M1662
Aragoni MC, Lippolis V, Mancini A, Pintus A, Podda E, Orton JB, Coles SJ, Arca M. 2-(5,6-Diphenyl-1,2,4-Triazin-3-yl)pyridinium Dichloroiodate (I). Molbank. 2023; 2023(2):M1662. https://doi.org/10.3390/M1662
Chicago/Turabian StyleAragoni, M. Carla, Vito Lippolis, Annalisa Mancini, Anna Pintus, Enrico Podda, James B. Orton, Simon J. Coles, and Massimiliano Arca. 2023. "2-(5,6-Diphenyl-1,2,4-Triazin-3-yl)pyridinium Dichloroiodate (I)" Molbank 2023, no. 2: M1662. https://doi.org/10.3390/M1662
APA StyleAragoni, M. C., Lippolis, V., Mancini, A., Pintus, A., Podda, E., Orton, J. B., Coles, S. J., & Arca, M. (2023). 2-(5,6-Diphenyl-1,2,4-Triazin-3-yl)pyridinium Dichloroiodate (I). Molbank, 2023(2), M1662. https://doi.org/10.3390/M1662








