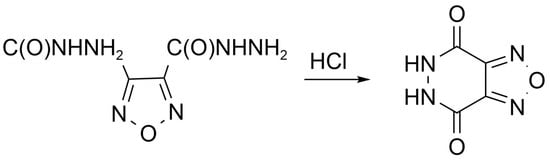
5,6-Dihydro-[1,2,5]oxadiazolo[3,4-d]pyridazine-4,7-dione
Abstract
1. Introduction
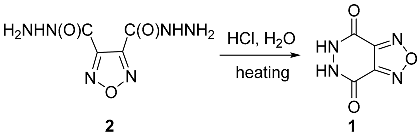
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ogurtsov, V.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Khrustalev, V.N.; Fakhrutdinov, A.N.; Zlotin, S.G.; Rakitin, O.A. [1,2,5]Oxadiazolo[3,4-d]pyridazine 1,5,6-trioxides: Efficient synthesis via the reaction of 3,4-bis(hydroxyimino)methyl)-1,2,5-oxadiazole 2-oxides with a mixture of concentrated nitric and trifluoroacetic acids and structural characterization. Tetrahedron Lett. 2018, 59, 3143–3146. [Google Scholar] [CrossRef]
- Kots, A.Y.; Khropov, Y.V.; Grafov, M.A.; Kulikov, A.S.; Ovchinnikov, I.V.; Belushkina, N.N.; Busygina, O.G.; Gavrilova, S.A.; Makhova, N.N.; Medvedeva, N.A.; et al. 1,2,5-Oxadiazolo[3,4-d]pyridazine-5,6-dioxide derivatives as activators of soluble form of guanylate-cyclase and agents active in the treatment of central nervous system diseases. Russian Patent RU2165256, 20 April 2001. [Google Scholar]
- Tang, Y.; He, C.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Energetic 1,2,5-Oxadiazolo-Pyridazine and its N-Oxide. Chem. A Eur. J. 2017, 23, 15022–15025. [Google Scholar] [CrossRef]
- Zheng, Y.; Qi, X.; Chen, S.; Song, S.; Zhang, Y.; Wang, K.; Zhang, Q. Self-Assembly of Nitrogen-Rich Heterocyclic Compounds with Oxidants for the Development of High-Energy Materials. ACS Appl. Mater. Interfaces 2021, 13, 28390–28397. [Google Scholar] [CrossRef]
- Gendron, D.; Morin, P.-O.; Najari, A.; Leclerc, M. Synthesis of New Pyridazine-Based Monomers and Related Polymers for Photovoltaic Applications. Macromol. Rapid Commun. 2010, 31, 1090–1094. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Gaisin, K.S.; Rakitin, O.A. 4,7-Bis(1,2,3,4,4a,9a-Hexahydro-9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine. Molbank 2021, 2021, M1295. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. 4,7-Dibromo-substituted 2,1,3-benzothia(selena,oxa)diazoles and [1,2,5]thia(selena)diazolo[3,4-c]pyridines as building blocks in solar cells components. Chem. Heterocycl. Comp. 2017, 53, 855–857. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Mikhalchenko, L.V.; Golovanov, I.S.; Amelichev, S.A.; Rakitin, O.A. Synthesis of 4,7-dibromo derivative of ultrahigh electron-deficient [1,2,5]thiadiazolo[3,4-d]pyridazine heterocycle and its cross-coupling reactions. Eur. J. Org. Chem. 2018, 41, 5668–5677. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Tanaka, E.; Popov, V.V.; Mikhalchenko, L.V.; Robertson, N.; Rakitin, O.A. [1,2,5]Thiadiazolo[3,4-d]pyridazine as an internal acceptor in the D-A-π-A organic sensitizers for dye-sensitized solar cells. Molecules 2019, 24, 1588. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, V.M.; Chmovzh, T.N.; Chkhetiani, G.R.; Taydakov, I.V.; Rakitin, O.A. New D–A–D luminophores of the [1,2,5]thiadiazolo[3,4-d]pyridazine series. Mendeleev Commun. 2022, 32, 371–373. [Google Scholar] [CrossRef]
- Eremeev, A.V.; Andrianov, V.G.; Piskunova, I.P. Reaction of functionally substituted vinyl ethers with 3,4-diaminofurazan and furazan-3,4-dicarboxylic acid dihydrazide. Chem. Heterocycl. Compd. 1979, 15, 261–264. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Lyssenko, K.A.; Popov, V.V.; Rakitin, O.A. Safe synthesis of 4,7-dibromo[1,2,5]thiadiazolo[3,4-d]pyridazine and its SNAr reactions. Molecules 2018, 23, 2576. [Google Scholar] [CrossRef] [PubMed]
 | |||
|---|---|---|---|
| Entry | Temperature, °C | Time, h | Yield, of 1, % |
| 1 | 75 | 3 | 15 |
| 2 | 75 | 2 | 25 |
| 3 | 75 | 1 | 41 |
| 4 | 60 | 1 | 39 |
| 5 | 90 | 1 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmovzh, T.N.; Gaisin, K.S.; Rakitin, O.A. 5,6-Dihydro-[1,2,5]oxadiazolo[3,4-d]pyridazine-4,7-dione. Molbank 2023, 2023, M1649. https://doi.org/10.3390/M1649
Chmovzh TN, Gaisin KS, Rakitin OA. 5,6-Dihydro-[1,2,5]oxadiazolo[3,4-d]pyridazine-4,7-dione. Molbank. 2023; 2023(2):M1649. https://doi.org/10.3390/M1649
Chicago/Turabian StyleChmovzh, Timofey N., Karim S. Gaisin, and Oleg A. Rakitin. 2023. "5,6-Dihydro-[1,2,5]oxadiazolo[3,4-d]pyridazine-4,7-dione" Molbank 2023, no. 2: M1649. https://doi.org/10.3390/M1649
APA StyleChmovzh, T. N., Gaisin, K. S., & Rakitin, O. A. (2023). 5,6-Dihydro-[1,2,5]oxadiazolo[3,4-d]pyridazine-4,7-dione. Molbank, 2023(2), M1649. https://doi.org/10.3390/M1649