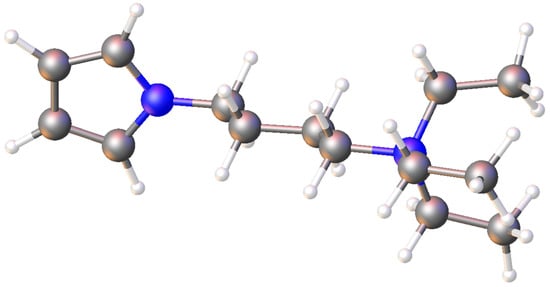
N,N,N-Triethyl-4-(1H-pyrrol-1-yl)butan-1-aminium Perchlorate
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Consideration
3.2. Synthesis of 1-(4-Bromobutyl)-1H-pyrrole 1
3.3. Synthesis of N,N,N-Triethyl-4-(1H-pyrrol-1-yl)butan-1-aminium Bromide 2
3.4. Synthesis of N,N,N-Triethyl-4-(1H-pyrrol-1-yl)butan-1-aminium Perchlorate 3
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lukyanov, D.A.; Vereshchagin, A.A.; Soloviova, A.V.; Grigorova, O.V.; Vlasov, P.S.; Levin, O.V. Sulfonated Polycatechol Immobilized in a Conductive Polymer for Enhanced Energy Storage. ACS Appl. Energy Mater. 2021, 4, 5070–5078. [Google Scholar] [CrossRef]
- Vereshchagin, A.A.; Potapenkov, V.V.; Vlasov, P.S.; Lukyanov, D.A.; Levin, O.V. Optimization of Sulfonated Polycatechol:PEDOT Energy Storage Performance by the Morphology Control. Nanomaterails 2022, 12, 1917. [Google Scholar] [CrossRef] [PubMed]
- Lukyanov, D.A.; Kalnin, A.Y.; Rubicheva, L.G.; Potapenkov, V.V.; Bakulina, O.Y.; Levin, O.V. Application of a TEMPO-Polypyrrole Polymer for NOx-Mediated Oxygen Electroreduction. Catalysts 2022, 12, 1466. [Google Scholar] [CrossRef]
- Pang, A.L.; Arsad, A.; Ahmadipour, M. Synthesis and factor affecting on the conductivity of polypyrrole: A short review. Polym. Adv. Technol. 2020, 32, 1428–1454. [Google Scholar] [CrossRef]
- Swager, T.M. 50th Anniversary Perspective: Conducting/Semiconducting Conjugated Polymers. A Personal Perspective on the Past and the Future. Macromolecules 2017, 50, 4867–4886. [Google Scholar] [CrossRef]
- Vereshchagin, A.A.; Lukyanov, D.A.; Kulikov, I.R.; Panjwani, N.A.; Alekseeva, E.A.; Behrends, J.; Levin, O.V. The Fast and the Capacious: A [Ni(Salen)]-TEMPO Redox-Conducting Polymer for Organic Batteries. Batteries Supercaps 2020, 4, 336–346. [Google Scholar] [CrossRef]
- Kulikov, I.; Panjwani, N.A.; Vereshchagin, A.A.; Spallek, D.; Lukianov, D.A.; Alekseeva, E.V.; Levin, O.V.; Behrends, J. Spins at work: Probing charging and discharging of organic radical batteries by electron paramagnetic resonance spectroscopy. Energy Environ. Sci. 2022, 15, 3275–3290. [Google Scholar] [CrossRef]
- Ouyang, J. Application of intrinsically conducting polymers in flexible electronics. SmartMat 2021, 2, 263–285. [Google Scholar] [CrossRef]
- Sengodu, P.; Deshmukh, A.D. Conducting polymers and their inorganic composites for advanced Li-ion batteries: A review. RSC Adv. 2015, 5, 42109–42130. [Google Scholar] [CrossRef]
- R. Murad, A.; Iraqi, A.; Aziz, S.B.; N. Abdullah, S.; Brza, M.A. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, A.; Han, Y.; Li, T. Sensors based on conductive polymers and their composites: A review. Polym. Int. 2019, 69, 7–17. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Alam, J.; Dass, L.A.; Raja, M. Recent advances in conjugated polymers for light emitting devices. Int. J. Mol. Sci. 2011, 12, 2036–2054. [Google Scholar] [CrossRef] [PubMed]
- Gautam, B.; Ayalew, H.; Dhawan, U.; Janardhanan, J.A.; Yu, H.-H. Layer-by-layer assembly and electrically controlled disassembly of water-soluble poly(3,4-ethylenedioxythiophene) derivatives for bioelectronic interface. J. Chin. Chem. Soc. 2020, 67, 1602–1610. [Google Scholar] [CrossRef]
- Amaya, T.; Hatai, T.; Kurata, I.; Hirao, T. Synthesis of self-doped polyaniline bearing phosphonic acid moiety via Pd-catalyzed phosphonation of poly(2-bromoaniline). Tetrahedron Lett. 2018, 59, 1715–1718. [Google Scholar] [CrossRef]
- Boullanger, S.; Contal, E.; Buron, C.C.; Viau, L. Pyrrole-tailed imidazolium surface-active monomers: Aggregation properties in aqueous solution and polymerization behavior. J. Mol. Liq. 2022, 350, 118588. [Google Scholar] [CrossRef]
- Riegel, N.; Darcel, C.; Stéphan, O.; Jugé, S. Mono and diphosphine borane complexes grafted on polypyrrole matrix: Direct use as supported ligands for Rh and Pd catalysis. J. Organometall. Chem. 1998, 567, 219–233. [Google Scholar] [CrossRef]
- Zhang, X.; Fried, A.; Knapp, S.; Goldman, A.S. Novel synthesis of enamines by iridium-catalyzed dehydrogenation of tertiary amines. Chem. Commun. 2003, 16, 2060–2061. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potapenkov, V.V.; Levin, O.V.; Lukyanov, D.A. N,N,N-Triethyl-4-(1H-pyrrol-1-yl)butan-1-aminium Perchlorate. Molbank 2023, 2023, M1643. https://doi.org/10.3390/M1643
Potapenkov VV, Levin OV, Lukyanov DA. N,N,N-Triethyl-4-(1H-pyrrol-1-yl)butan-1-aminium Perchlorate. Molbank. 2023; 2023(2):M1643. https://doi.org/10.3390/M1643
Chicago/Turabian StylePotapenkov, Vasiliy V., Oleg V. Levin, and Daniil A. Lukyanov. 2023. "N,N,N-Triethyl-4-(1H-pyrrol-1-yl)butan-1-aminium Perchlorate" Molbank 2023, no. 2: M1643. https://doi.org/10.3390/M1643
APA StylePotapenkov, V. V., Levin, O. V., & Lukyanov, D. A. (2023). N,N,N-Triethyl-4-(1H-pyrrol-1-yl)butan-1-aminium Perchlorate. Molbank, 2023(2), M1643. https://doi.org/10.3390/M1643