Methyl 3,3-Bis[4-(dimethylamino)phenyl]-2,2-dimethylpropanoate
Abstract
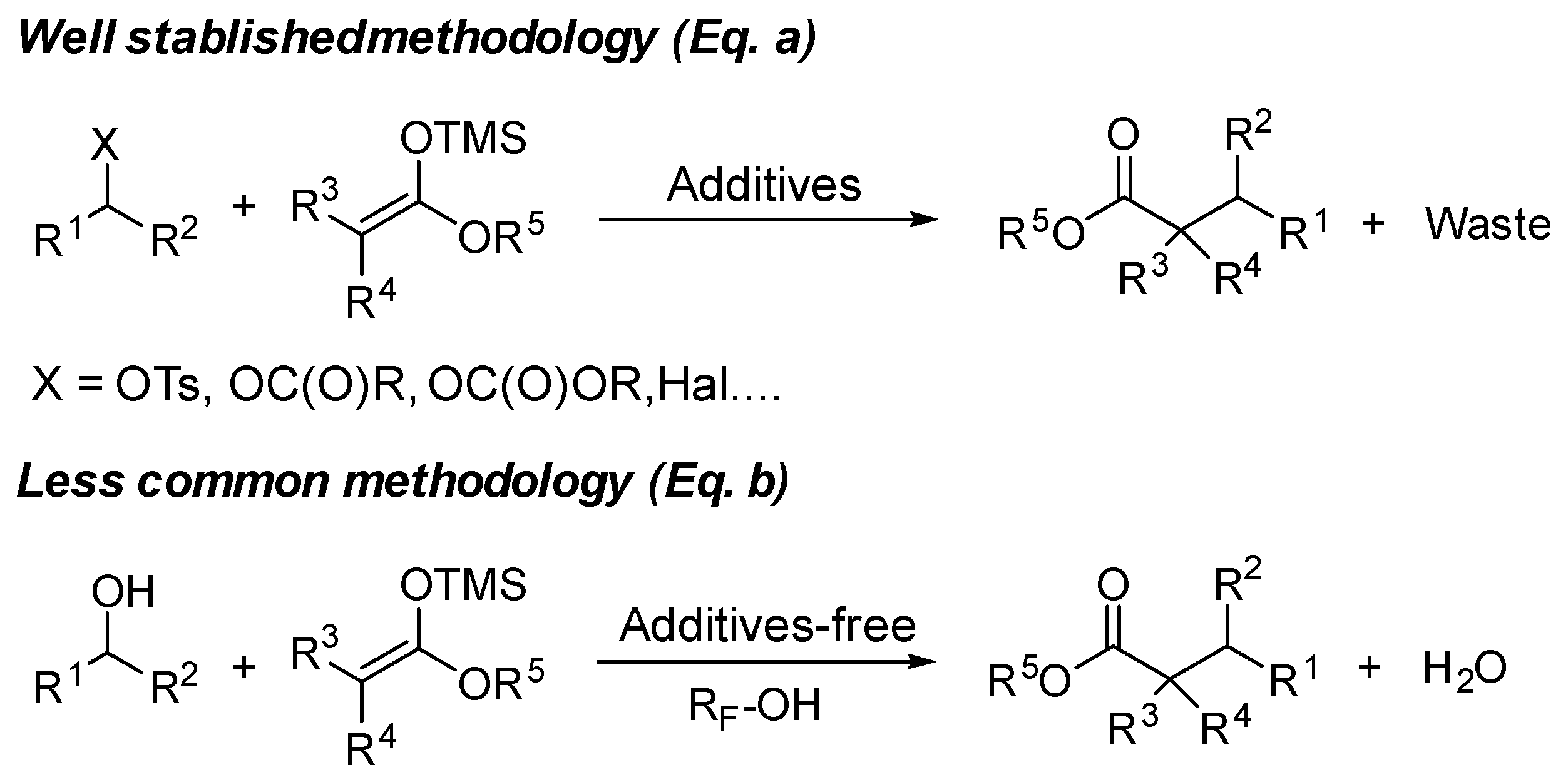
1. Introduction
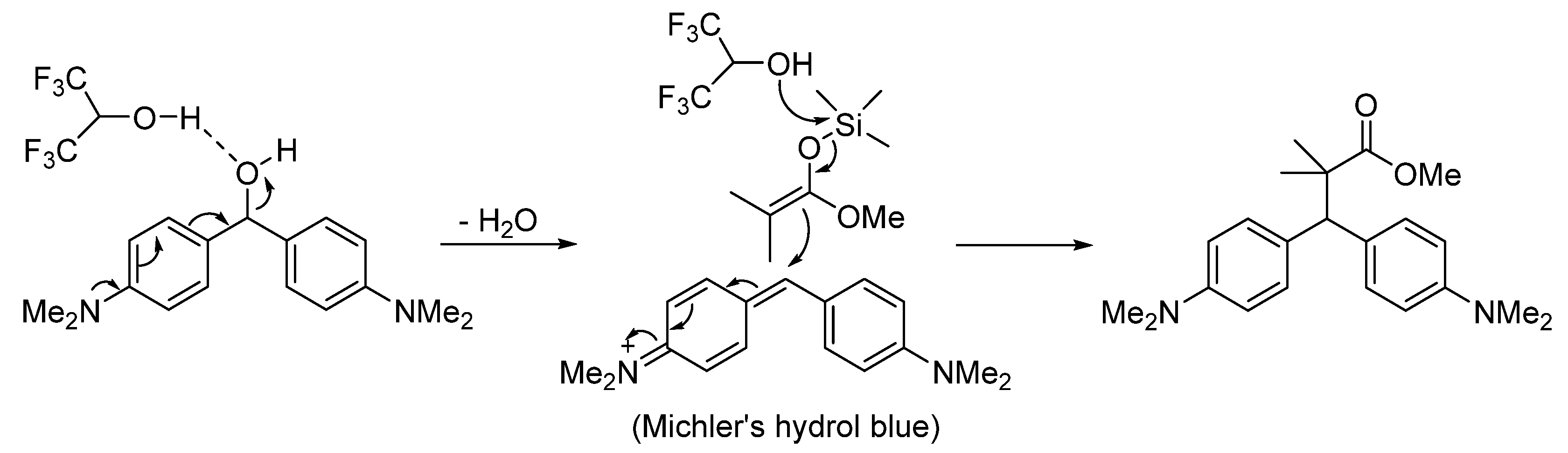
2. Results
3. Materials and Methods
General Procedure for the HFIP-Promoted Synthesis of Ester 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baeza, A.; Nájera, C. Recent Advances in the Direct Nucleophilic Substitution of Allylic Alcohols trough SN1-Type Reactions. Synthesis 2014, 46, 25–34. [Google Scholar] [CrossRef]
- Llopis, N.; Baeza, A. HFIP-Promoted Synthesis of Substituted Tetrahydrofurans by Reaction of Epoxides with Electron-Rich Alkenes. Molecules 2020, 25, 3464–3476. [Google Scholar] [CrossRef] [PubMed]
- Llopis, N.; Baeza, A. Oxidation of Electron-Rich Arenes Using HFIP-UHP System. J. Org. Chem. 2020, 85, 6159–6164. [Google Scholar] [CrossRef] [PubMed]
- Llopis, N.; Gisbert, P.; Baeza, A. Direct Synthesis of N,N-Disubstituted Formamides by Oxidation of Imines Using an HFIP/UHP System. J. Org. Chem. 2020, 85, 11072–11079. [Google Scholar] [CrossRef] [PubMed]
- Llopis, N.; Gisbert, P.; Baeza, A. Oxidative Cleavage of Indoles Mediated by Urea Hydrogen Peroxide or H2O2 in Polar Solvents. Adv. Synth. Catal. 2021, 363, 3245–3249. [Google Scholar] [CrossRef]
- Llopis, N.; Gisbert, P.; Baeza, A.; Correa-Campillo, J. Dehydrogenation of N-Heterocyclic Compounds Using H2O2 and Mediated by Polar Solvents. Adv. Synth. Catal. 2022, 364, 1205–1210. [Google Scholar] [CrossRef]
- Colomer, I.; Chamberlain, A.E.R.; Haughey, M.B.; Donohoe, T.J. Hexafluoroisopropanol as a highly versatile solvent. Nat. Rev. Chem. 2017, 1, 0088. [Google Scholar] [CrossRef]
- An, X.-D.; Xiao, J. Fluorinated Alcohols: Magic Reaction Medium and Promoters for Organic Synthesis. Chem. Rec. 2020, 20, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Motiwala, H.F.; Armaly, A.M.; Cacioppo, J.G.; Coombs, T.C.; Koehn, K.R.K.; Norwood, V.M., IV; Aubé, J. HFIP in Organic Synthesis. Chem. Rev. 2022, 122, 12544–12747. [Google Scholar] [CrossRef] [PubMed]
- Trillo, P.; Baeza, A.; Nájera, C. Fluorinated Alcohols as Promoters for the Metal-Free Direct Substitution Reaction of Allylic Alcohols with Nitrogenated, Silylated, and Carbon Nucleophiles. J. Org. Chem. 2012, 77, 7344–7354. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.M.; Maquilón, C.; Ramón, D.J.; Baeza, A. Hexafluoroisopropanol-Promoted Metal-Free Allylation of Silyl Enol Ethers with Allylic Alcohols. Asian J. Org. Chem. 2017, 6, 1440–1444. [Google Scholar] [CrossRef]
- Trillo, P.; Baeza, A.; Nájera, C. Bis(2-aminobenzoimidazole)-Organocatalyzed Asymmetric Alkylation of Activated Methylene Compounds with Benzylic and Allylic Alcohols. Synthesis 2014, 46, 3399–3407. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mollà-Guerola, L.; Baeza, A. Methyl 3,3-Bis[4-(dimethylamino)phenyl]-2,2-dimethylpropanoate. Molbank 2023, 2023, M1642. https://doi.org/10.3390/M1642
Mollà-Guerola L, Baeza A. Methyl 3,3-Bis[4-(dimethylamino)phenyl]-2,2-dimethylpropanoate. Molbank. 2023; 2023(2):M1642. https://doi.org/10.3390/M1642
Chicago/Turabian StyleMollà-Guerola, Lara, and Alejandro Baeza. 2023. "Methyl 3,3-Bis[4-(dimethylamino)phenyl]-2,2-dimethylpropanoate" Molbank 2023, no. 2: M1642. https://doi.org/10.3390/M1642
APA StyleMollà-Guerola, L., & Baeza, A. (2023). Methyl 3,3-Bis[4-(dimethylamino)phenyl]-2,2-dimethylpropanoate. Molbank, 2023(2), M1642. https://doi.org/10.3390/M1642





