1. Introduction
Non-symmetrically di-aryl substituted carbenium salts comprise an important class of organic synthetic intermediates, which react with a wide range of neutral π-, n-, σ-, or carbanion nucleophiles, resulting in tetrahedral sp
3-carbon centered tri-aryl methane compounds [
1]. Indole-3-ylmethylium tetrafluoroborates are easily accessible, highly stable, and have a wide range of synthetic applications in organic chemistry [
2]. These Lewis acids, as well as electrophilic reaction partners, were extensively utilized in a variety of conversions, including the enantioselective synthesis of unsymmetrical tri-aryl methanes [
3], synthesis of 2,3-disubstituted indolines [
4], and intramolecular tandem reactions [
5].
The phosphine-based ligand 1,3,5-triaza-7-phosphaadmantane (PTA), which is also a Lewis base, is frequently used in organometallic chemistry, homogeneous transition metal catalysis, and organocatalytic methods [
6]. Because of its three tertiary amine functions, it can be easily incorporated into hydrogen-bonding networks and is easily solvated. When exposed to acids or alkyl halides, the molecule acts as a strong nucleophile, forming both phosphonium and ammonium cations. PTA-phosphonium salts gained importance, since the reductive cleavage of these PTA–cage-phosphonium salts produced novel, helmet-shaped P, N-bidentate ligands [
7]. The general synthesis of PTA-phosphonium salts involves unstable P-alkylated primary phosphanes or alkyltris (hydroxy-methyl) phosphonium salts as starting materials [
7]. However, these methods are hampered by limitations such as poor reagent modification options and poor yields. Considering all these challenges, an experimental investigation was carried out with regard to the Lewis base reactivity of PTA [
8] to be utilized towards bench stable carbenium salts, specifically targeting novel PTA-derived di-aryl-methyl (or aryl-indol-3-methyl) phosphonium salts. These intermediates may be further used as carbocation sources [
9] and potentially lead to new P, N-type organometallic ligands under Na/NH
3 (liq.) conditions via PTA-cage cleavage [
7]. Furthermore, considering that PTA and its sultanate-derivative salts, PTABS and PTAPS, are highly water soluble, it was anticipated that the resulting PTA-di-aryl-methyl phosphonium salts will be as well [
6,
10,
11,
12]. To initiate a respective study, the synthesis of 7-((5-bromo-1H-indol-3-yl)(4-methoxyphenyl)methyl)-1,3,5-triaza-7-phosphaadamantan-7-ium tetrafluoroborate was investigated, with its synthesis and comprehensive characterization being reported here.
2. Results and Discussion
In previous reports, the indole-3-ylmethylium ion (
3) was demonstrated to exclusively react towards π-nucleophiles, n-nucleophiles, phosphines, and pyridine Lewis bases with complete selectivity towards the C-10 position and with high yields of the resulting products [
9,
13]. Inspired by these reports, it was decided to look into the reaction of the indole-3-ylmethylium ion (
3) with the phosphine Lewis base 1,3,5-triaza-7-phosphaadmantane (PTA).
We successfully synthesized 7-((5-bromo-1H-indol-3-yl)(4-methoxyphenyl)methyl)-1,3,5-triaza-7-phosphaadamantan-7-ium tetrafluoroborate (
4) from PTA, 4-methoxy benzaldehyde (
2), and 5-bromo indole (
1) (
Scheme 1). Aryl(indole-3-yl)methylium tetrafluoroborate (
3) was first prepared in a single pot reaction by adding 1.5 equivalents of HBF
4·OEt
2 to a 1:1 mixture of 5-bromoindole (
1) and 4-methoxy benzaldehyde (
2) in a 1:1 CH
2Cl
2:Et
2O solution (
Scheme 1) [
2,
13]. In addition,
3 precipitated from the solution as a bench-stable, long-lasting bright red solid in essentially quantitative yields (> 95%). Spectroscopic methods were used to confirm the chemical structure of the product (
3), and the results were consistent with the data that had already been published [
2]. Compound
3 was then dissolved in a 1:3 CH
2Cl
2:CH
3COCH
3 solution of 1 equivalent of 1,3,5-triaza-7-phosphaadmantane (PTA). Within 5 min, the dark-red solution lost its color and became colorless instead. Upon the addition of excess diethyl ether, a pale pink colored solid precipitated from the solution. This was filtered, washed, and dried under reduced pressure. The crude product
4 was obtained in 77% yield and then further purified by recrystallizing from hot acetone, yielding colorless shiny needles.
The chemical and molecular structures of this product, being the title compound 4, were confirmed by 1D–NMR (1H, 13C, 31P, 19F, and 11B), ESI–MS, elemental analysis, and a single crystal X-ray diffraction analysis.
The
1HNMR spectrum of
3 interestingly implies a ca. 5:1 (
Z):(
E) diastereomeric mixture in CD
3CN solution. It was previously pointed out that the distribution of the (
Z) and (
E) isomers in CD
3CN is independent of the temperature, and that the isomeric interconversion on the NMR time scale is not obvious [
7]. In the
1HNMR spectrum of the novel PTA-di-aryl phosphonium salt
4, the singlet at 8.9 ppm for the H-10 atom in compound
3 has vanished, verifying that the functionalization of the sp
2-carbon-10 has indeed taken place. The H-10 chemical shift in Product
4, which now binds to a sp
3-carbon atom, appears upfield at 5.7 ppm. Additionally, the establishment of the C–P bond at the C–10 position is confirmed by the respective
2JH-P coupling at 20.6 Hz with a well-defined doublet. The C–10 functionalization is further supported by the
13C NMR spectra of
4, where the carbon–10 exhibits a chemical shift of 46.6 ppm and a
1JC-P coupling constant of 26.6 Hz. As compared to the -101 ppm chemical shift of free PTA, the phosphonium salt
4 gives a downfield
31P NMR signal at −43.3 ppm. In addition, the presence of
4 as a BF
4– salt in DMSO solution was confirmed by the
11B NMR signal at −1.29 ppm and the
19F NMR signal at −148.8 ppm. The [(M − BF
4¯) + H] peak in the ESI mass spectrum at 473.5 m/z, and a few other reasonable fragmentation peaks (see
Supplementary Materials) further confirm the composition of
4. Finally, the molecular structure of compound
4 was determined by a single crystal X-ray diffraction analysis. Repeated recrystallization of the product from hot acetone (specifically: hot filtration and slow evaporation) yielded eventually large-enough colorless needles of
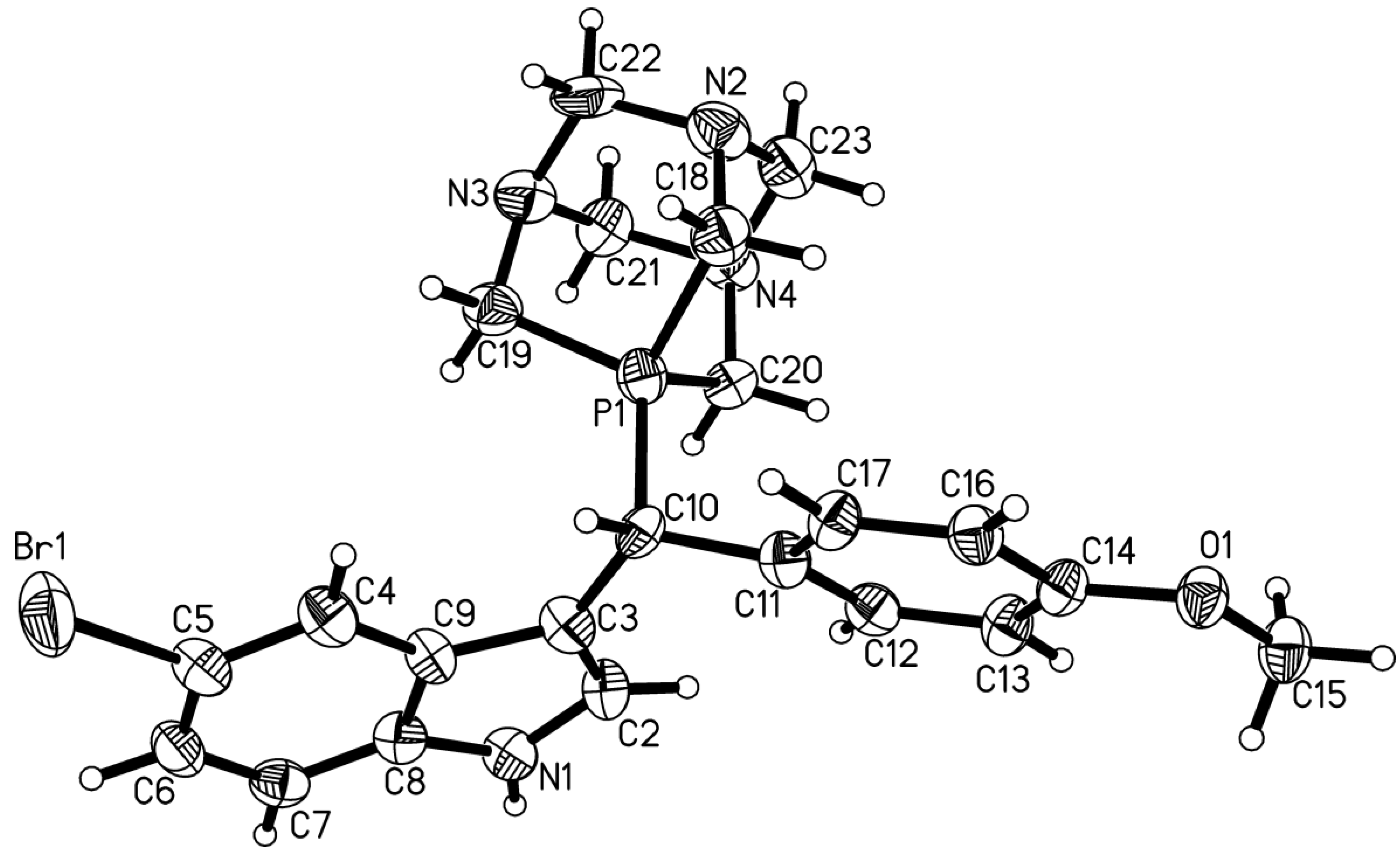
4. The molecular structure of the cation of
4 is shown in
Figure 1. A metrical analysis of the X-ray data revealed that the indole ring’s C2-C3 double bond is shorter (1.382 Å) compared to the published data for the indol-3-ylmethylium tetrafluoroborate salt
3 (1.410 Å), in which the carbenium center C–10 is in conjugation with the indole C2-C3 bond, thereby reducing its double bond character [
13].
Notably, the bond lengths of the central carbon C10 to the indole and to the phenyl moieties differ, with the former being shorter (C3-C10 1.504 Å) than the latter (C10-C11 1.520 Å). Other metrical parameters are in accordance with the usually observed ones for the individual moieties of the molecule.
Considering the aim of accessing water-soluble PTA derivatives, the aqueous solubility of 4 was tested. Surprisingly, 4 is only poorly soluble at room temperature. The solubility of 4 in water improved at 80 °C, but for a cost. A respective 31P NMR analysis of the resultant solution in D2O revealed the presence of decomposition products besides 4. The identification of these byproducts is currently being investigated.
3. Materials and Methods
5-Bromoindole [CAS No. 10075-50-0] and 4-methoxy benzaldehyde [CAS No. 123-11-5] were purchased from Acros Organics, Nidderau, Germany. The tetrafluoroboric acid–diethyl ether complex [CAS No. 67969-82-8] was purchased from Sigma–Aldrich, Merck (Darmstadt, Germany). 1,3,5-Triaza-7-phosphaadmantane (PTA) was synthesized according to the literature in gram scale [
14]. The solvents dichloromethane and diethyl ether were used as received.
Unless otherwise mentioned, all reactions were performed under nitrogen atmosphere using oven-dried standard Schlenk glassware. 1H NMR (300 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker Avance II–300 spectrometer(Rheinstetten, Germany). Chemical shifts δ are given in ppm and the solvent residual peak (CDCl3: 1H, δ = 7.27; 13C, δ = 77.0 and DMSO-d6: 1H, δ = 2.50; 13C, δ = 40) was used as an internal standard. Peak multiplicities are specified as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad. Mass spectra (m/z) were recorded on a compact ESI-mass spectrometer (Harlow, UK)Advion expression CMS. Mechenary–Nagel silica gel 60 F254 plates were used for thin layer chromatography (TLC), and detection was achieved with UV light. An Elementar Vario MICRO cube (Langenselbold, Germany)was used for the experimental determination of elemental compositions of the final pure products.
Compound
3 (
Figure 2) was synthesized according to the literature procedure [
13]. Benzaldehyde
2 (0.5 mL, 1 equiv.) was dissolved in a combination of CH
2Cl
2 (5 mL) and Et
2O (5 mL) in a flame-dried Schlenk flask flushed with nitrogen. Then, 5-bromo indole
1 (0.687 g, 1 equiv.) was added, and the mixture was stirred until fully homogenized (2–5 min). HBF
4·OEt
2 (0.714 mL, 1.50 equiv.) was added dropwise after the solution had been chilled to 0 °C. A brightly red-colored solid was precipitated after 10–15 min while the solution warmed up to room temperature. After 15 min, the material was filtered, thoroughly washed with Et
2O (4 × 25 mL) and crystallized from CH
3CN:Et
2O (1:1) to give dark magenta-red crystals of high purity in quantitative yield. The major isomer in the product is (
Z)-5-bromo-3-(4-methoxybenzylidene)-3H-indol-1-ium tetrafluoroborate.
The spectroscopic data are in agreement with literature data: 1H NMR (300 MHz, CD3CN) δ: 9.13 (d, 1H, J = 0.9 Hz, 2-H), 8.91 (s, 1H, 10-H), 8.12−8.05 (m, 3H, 4-H, 12-H and 17-H), 7.78−7.71 (m, 1H, 7-H), 7.70−7.61 (m, 1H, 6-H), 7.22 (d, 2H, J = 8.9 Hz, 13-H and 16-H), 3.99 (s, 3H, 15-H). 13C NMR (75MHz, CD3CN) δ: 57.21 (s, 1 C) 117.90 (s, 1 C) 124.35 (s, 1 C) 127.92 (s, 1 C) 131.67 (s, 1 C) 139.80 (s, 1 C) 152.03 (s, 1 C) 162.71 (s, 1 C); 19F NMR (282 MHz, CD3CN) δ: −151.7. ESI-MS m/z: [M − BF4−]+ Calcd. for C16H13BrNO2+: 315.19; Found: [(M − BF4−) +H)+ 316.2.
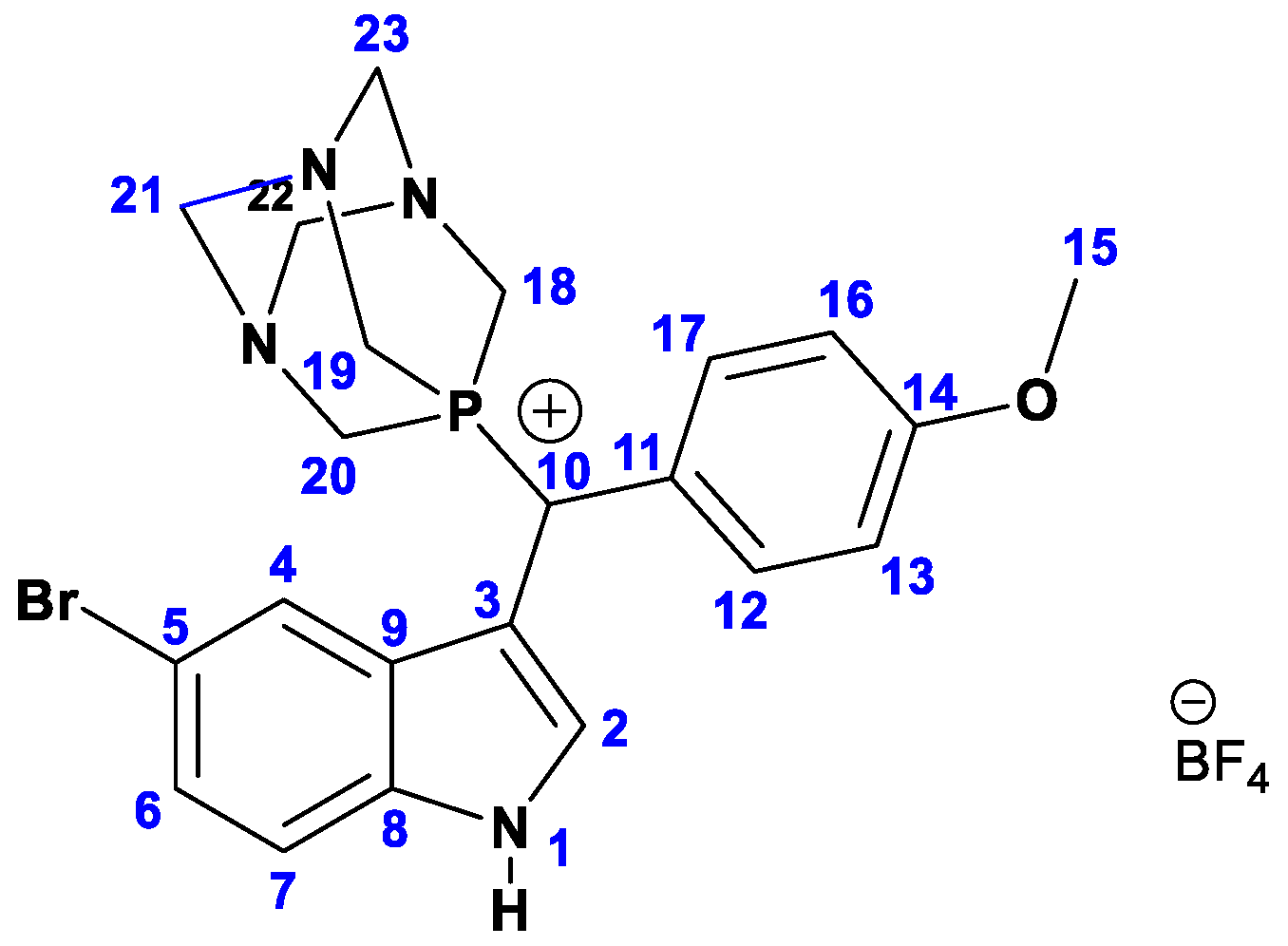
Figure 2.
Atom numbering for the NMR assignment for 3.
Figure 2.
Atom numbering for the NMR assignment for 3.
The synthetic process was adapted from the literature with a few minor changes applied for a higher isolated yield [
9]. 1,3,5-Triaza-7-phosphaadamantan (39.3 mg, 0.25 mmol, 1.0 equiv.) was added to a dark, orange-colored solution of
3 (0.1 g, 0.25 mmol, 1.00 equiv.) in a CH
2Cl
2:CH
3COCH
3 (1:3) solvent mixture, upon which the color instantly vanished. The solution was agitated for 5 min at 20 °C. Upon the slow addition of Et
2O (10 mL),
4 precipitated as a light-pink solid (0.107 g, 0.192 mmol, 77%) (
Figure 3).
m.p. 208–210 °C. 1H NMR (300 MHz, DMSO (d6)) δ: 11.77 (s, 1H, NH), 7.96 (t, J = 2.4 Hz, 1H, 4-H), 7.80 (d, J = 1.7 Hz, 1H, 2-H), 7.41 (dd, J = 8.7, 2.6 Hz, 3H,12-H,17-H, 6H), 7.28 (dd, J = 8.6, 1.8 Hz, 1H, 7-H), 7.02 (d, J = 8.6 Hz, 2H, 13-H, 16-H), 5.70 (d, 2JH,P = 20.6 Hz, 1H, 10-H), 4.50–4.29 (m, 12H, 2 × 18-H, 2 × 19-H, 2 × 20-H, 2 × 21-H, 2 × 22-H, 2 × 23-H), 3.77 (s, 3H, 15-H). 13C NMR (75 MHz, DMSO (d6)) δ: 159.77 (s), 130.54 (d, J = 5.9 Hz), 128.63–128.48 (m), 127.06 (s), 123.47 (s), 115.42 (s), 71.01 (d, J = 9.5 Hz), 46.64 (d, J = 26.6 Hz).;31P NMR (121 MHz, DMSO (d6)) δ: −43.05; 19F NMR (282 MHz, DMSO(d6)) δ: −148.18, −148.23; 11B NMR (96 MHz, DMSO(d6)) δ: −1.29; ESI-MS: [M − BF4−]+ Calcd for C22H25BrN4OP 472.35; Found 473.5 [(M − BF4−) + H)]. Anal. Calcd for C22H25BBrF4N4OP: C, 47.26; H, 4.51; N, 10.02, found: C, 47.13; H, 4.29; N, 9.95. To obtain crystals suitable for a single crystal X-ray diffraction analysis, the product was recrystallized from hot acetone.
Figure 3.
Atom numbering for the NMR assignment for 4.
Figure 3.
Atom numbering for the NMR assignment for 4.
Diffraction data were collected at low temperature (−173.0 °C) using a Rigaku–XtaLAB Synergy-S/
i diffractometer with a microfocus copper source and copper Kα radiation, λ = 1.54184 Å. The structure was solved with SHELXT–2018 and refined by the full-matrix least-squares technique (SHELXL–2018) using the WINGX GUI [
15,
16,
17]. All non-hydrogen atoms were refined with anisotropic displacement parameters. The N-bound hydrogen atom on N1 was located and refined freely. All other hydrogen atoms were refined isotropically on calculated positions using a riding model with their
Uiso values constrained to 1.5
Ueq of their pivot atoms for terminal sp
3 carbon atoms and 1.2 times for the aromatic, methylene, and methine carbon atoms. General crystallographic, crystal, and refinement data along with a full set of metrical parameters for
4 are provided in the
Supplementary Materials file. Crystallographic data were deposited with the Cambridge Crystallographic Data Centre, CCDC, 12 Union Road, Cambridge CB21EZ, UK. These data can be obtained free of charge on quoting the depository number CCDC 2250409 by FAX (+44-1223-336-033), email (
deposit@ccdc.cam.ac.uk) or their web interface (at
http://www.ccdc.cam.ac.uk).