Synthesis and X-ray Structures of Potential Light-Harvesting Ruthenium(II) Complexes
Abstract
1. Introduction
2. Results and Discussion
2.1. NMR Spectroscopy
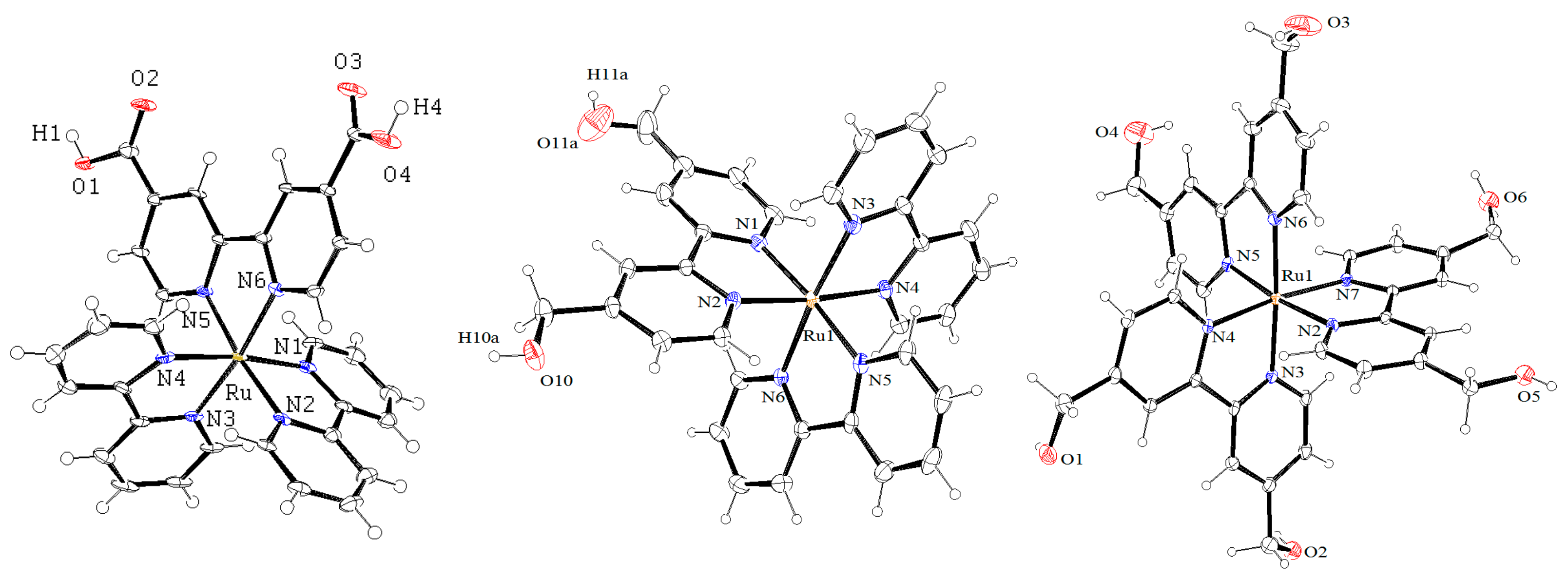
2.2. Crystallization and Structure Determination
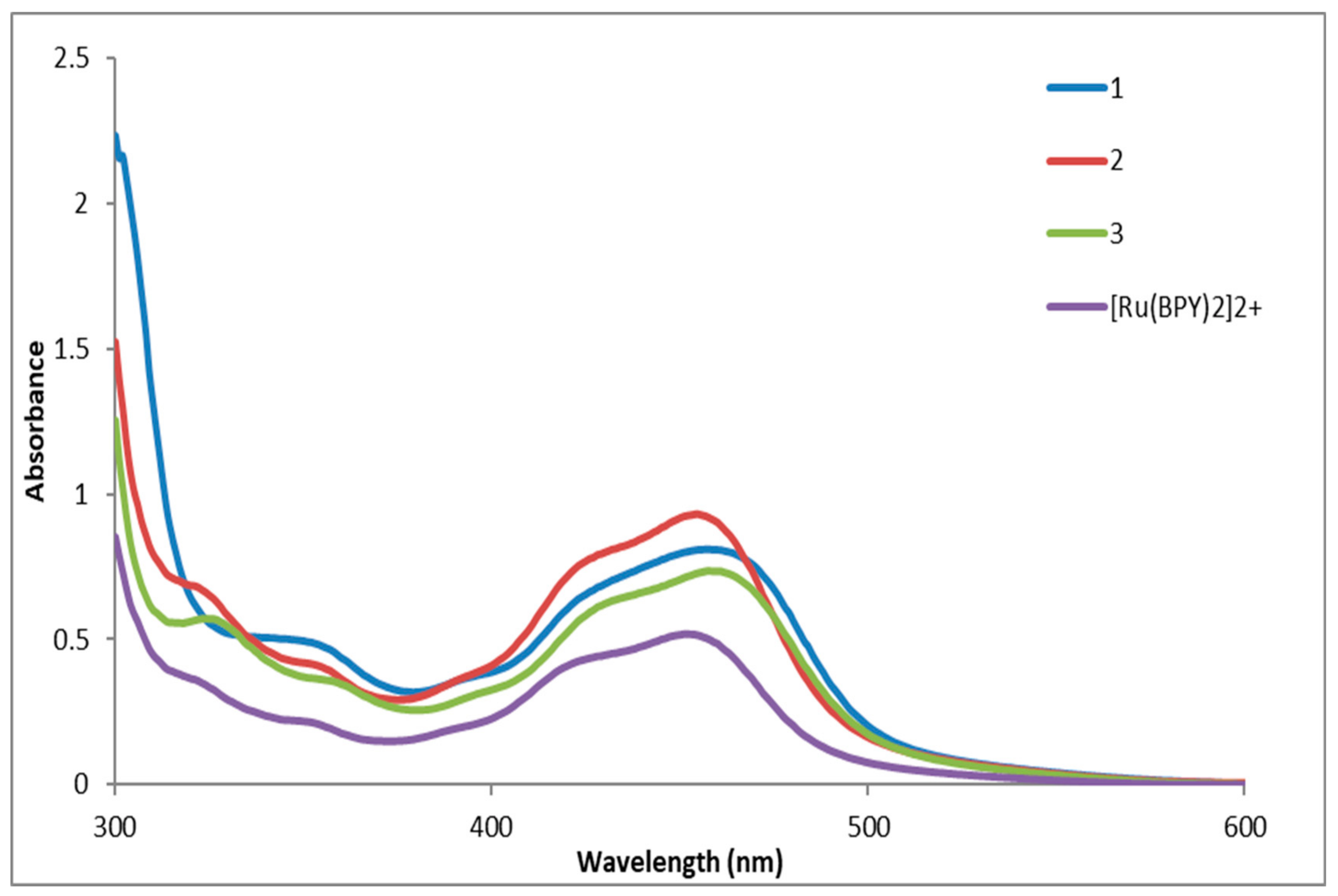
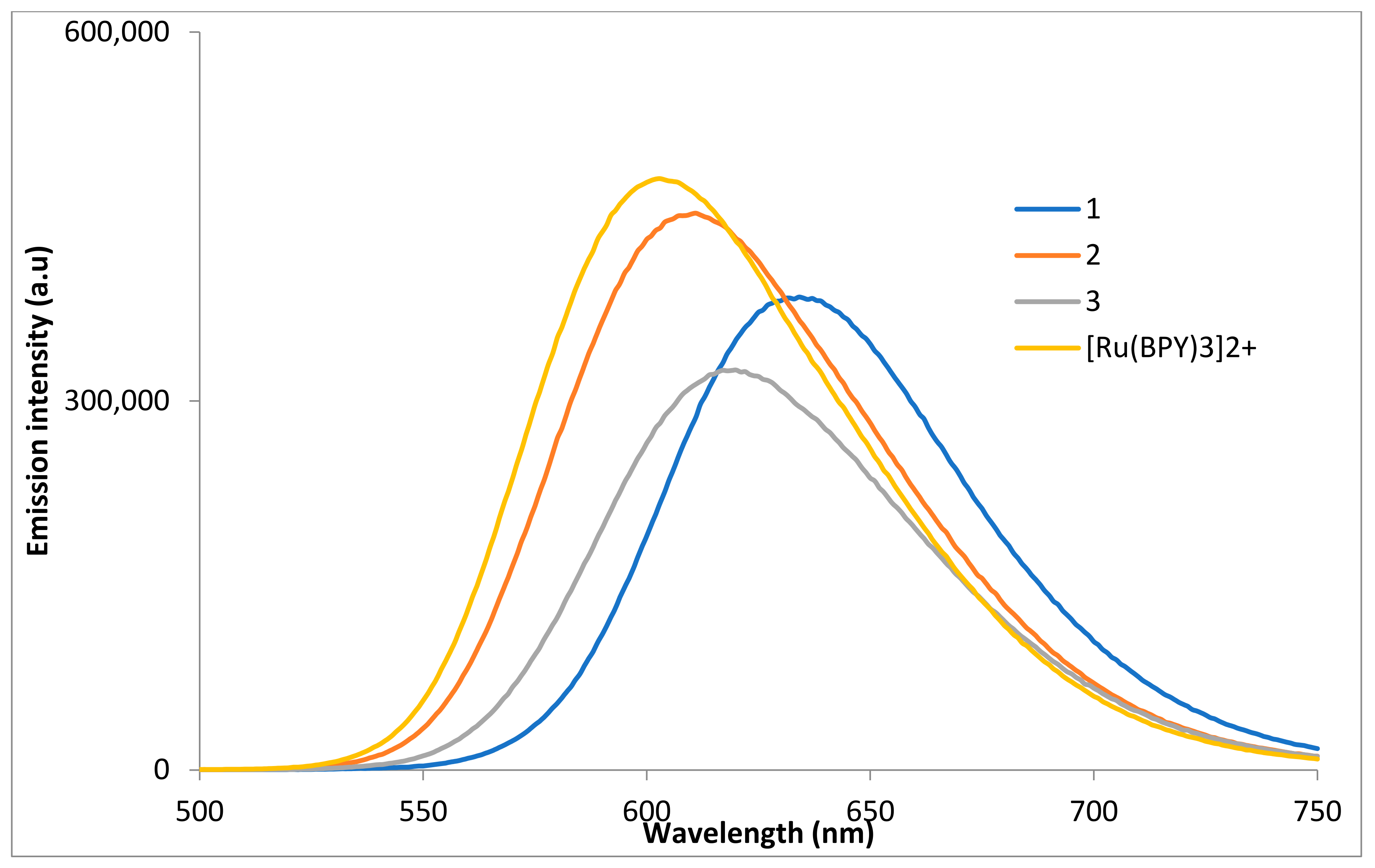
2.3. Optical Properties
2.3.1. Absorbance Spectroscopy
2.3.2. Emission Spectroscopy
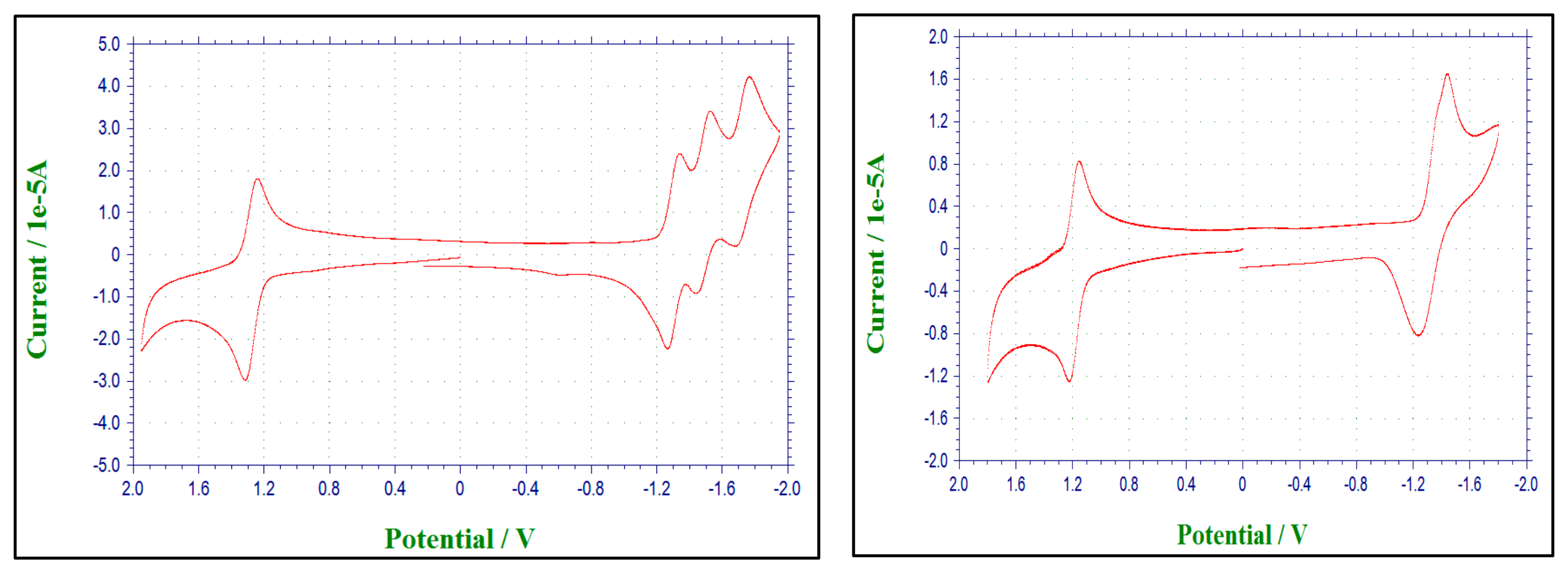
2.4. Cyclic Voltammetry
3. Materials and Methods
3.1. Materials
3.2. Physical Measurements
3.3. Single-Crystal X-ray Structure Determination
3.4. Experimental Procedure
Synthesis of Ruthenium(II) Complexes (1–3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lior, N. Energy resources and use: The present situation and possible paths to the future. Energy 2008, 33, 842–857. [Google Scholar] [CrossRef]
- Qin, Y.; Peng, Q. Ruthenium sensitizers and their applications in dye-sensitized solar cells. Int. J. Photoenergy 2012, 2012, 842–857. [Google Scholar] [CrossRef]
- Kohle, O.; Grätzel, M.; Meyer, A.F.; Meyer, T.B. The photovoltaic stability of, bis (isothiocyanato) rutheniurn (II)-bis-2, 2′ bipyridine-4, 4′-dicarboxylic acid and related sensitizers. Adv. Mater. 1997, 9, 904–906. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Pechy, P.; Renouard, T.; Zakeeruddin, S.M.; Humphry-Baker, R.; Comte, P.; Liska, P.; Cevey, L.; Costa, E.; Shklover, V. Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2-based solar cells. J. Am. Chem. Soc. 2001, 123, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Beauvilliers, E.E.; Meyer, G.J. Evidence for Cation-Controlled Excited-State Localization in a Ruthenium Polypyridyl Compound. Inorg. Chem. 2016, 55, 7517–7526. [Google Scholar] [CrossRef] [PubMed]
- Caspar, R.; Amouri, H.; Gruselle, M.; Cordier, C.; Malézieux, B.; Duval, R.; Leveque, H. Efficient asymmetric synthesis of Δ-and Λ-enantiomers of (bipyridyl) ruthenium complexes and crystallographic analysis of Δ-bis (2, 2′-bipyridine)(2, 2′-bipyridine-4, 4′-dicarboxylato) ruthenium: Diastereoselective homo-and heterochiral ion pairing revisited. Eur. J. Inorg. Chem. 2003, 2003, 499–505. [Google Scholar]
- Pang, J.; Di, Z.; Qin, J.-S.; Yuan, S.; Lollar, C.T.; Li, J.; Zhang, P.; Wu, M.; Yuan, D.; Hong, M. Precisely embedding active sites into a mesoporous Zr-framework through linker installation for high-efficiency photocatalysis. J. Am. Chem. Soc. 2020, 142, 15020–15026. [Google Scholar] [CrossRef] [PubMed]
- Guillo, P.; Hamelin, O.; Pécaut, J.; Ménage, S. Complexation to [Ru (bpy)2]2+: The trick to functionalize 3, 3′-disubstituted-2, 2′-bipyridine. Tetrahedron Lett. 2013, 54, 840–842. [Google Scholar] [CrossRef]
- Rillema, D.P.; Jones, D.S. Structure of tris (2, 2′-bipyridyl) ruthenium (II) hexafluorophosphate, [Ru(bipy)3][PF6]2; X-ray crystallographic determination. J. Chem. Soc. Chem. Commun. 1979, 19, 849–851. [Google Scholar] [CrossRef]
- Hansen, L.E.; Glowacki, E.R.; Arnold, D.L.; Bernt, G.J.; Chi, B.; Fites, R.J.; Freeburg, R.A.; Rothschild, R.F.; Krieg, M.C.; Howard, W.A. Syntheses and characterization of some chloro, methoxy, and mercapto derivatives of [Ru (η2-2, 2′-bipyridine)3]2+2PF6−: Crystal and molecular structures of [Ru (η2-2, 2′-bipyridine) 2 (η2-4, 4′-(X) 2-2, 2′-bipyridine)]2+ 2PF6−(X= Cl, OCH3). Inorg. Chim. Acta 2003, 348, 91–96. [Google Scholar] [CrossRef]
- Rillema, D.P.; Allen, G.; Meyer, T.; Conrad, D. Redox properties of ruthenium (II) tris chelate complexes containing the ligands 2, 2’-bipyrazine, 2, 2’-bipyridine, and 2, 2’-bipyrimidine. Inorg. Chem. 1983, 22, 1617–1622. [Google Scholar] [CrossRef]
- Fuentes, M.J.; Bognanno, R.J.; Dougherty, W.G.; Boyko, W.J.; Kassel, W.S.; Dudley, T.J.; Paul, J.J. Structural, electronic and acid/base properties of [Ru (bpy (OH)2)3]2+(bpy(OH)2= 4, 4′-dihydroxy-2, 2′-bipyridine). Dalton Trans. 2012, 41, 12514–12523. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97. Program for Crystal Structure Refinement; ScienceOpen, Inc.: Burlington, MA, USA, 1997. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]

| Compounds | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C38H33Cl2N9O12Ru | C32H28Cl2N6O10Ru | C76H78N14O31Ru2Cl4 |
| Formula weight | 979.70 | 828.29 | 2027.46 |
| Wavelength | MoKα 0.71073 | MoKα 0.71073 | MoKα 0.71073 |
| System | SMART APEXII | SMART APEXII | SMART APEXII |
| Temperature, K | 100(2) | 100(2) | 100(2) |
| Crystal system | triclinic | monoclinic | Triclinic |
| Space group | P-1 | P 1 21/c 1 | P-1 |
| a, Å | 8.993(3) | 8.8451(6) | 10.7926(4) |
| b, Å | 14.987(5) | 30.857(2) | 11.1969(4) |
| c, Å | 15.291(5) | 14.0432(9) | 19.3405(8) |
| α, ° | 93.301(4) | 90 | 84.03 |
| β, ° | 93.474(4) | 99.2170(10) | 80.87 |
| γ, ° | 97.352(4) | 90 | 62.94 |
| Volume, Å3 | 2035.8(12) | 3783.4(4) | 2053.54(14) |
| Z | 2 | 8 | 1 |
| Density (calc) g·cm−3 | 1.598 | 1.441 | 1.639 |
| Absorb. Coef. Mm−1 | 0.591 | 0.615 | 1036 |
| F(000) | 996 | 1663 | 1036 |
| θ range | 2.51–24.08 | 2.42–24.86 | 2.26–27.27 |
| Index ranges | ±10, ±17, ±17 | ±10, ±37, ±16 | ±13, ±14, ±24 |
| Reflections collected | 18331 | 38991 | 24520 |
| Independent reflections | 6538 | 6986 | 9261 |
| Observed reflections | 5311 | 5610 | 7809 |
| Max/Min trans. | 0.737–0.943 | 0.866–0.943 | |
| Data/restr./param. | 6538/0/562 | 6986/2/462 | 9261/0/582 |
| Goodness-of-fit | 1.067 | 1.107 | 1.071 |
| Final R indices [I > 2σ(I)] | 0.0390 | 0.0522 | 0.0623 |
| R indices (all data) | 0.0539 | 0.0640 | 0.0751 |
| CCDC Number | 1443902 | 1857593 | 1857586 |
| 1 | 2 | 3 | |
|---|---|---|---|
| C=O (double bond) | 1.198 Å; 1.199 Å | - | - |
| C-O (single bond) | 1.311 Å; 1.346 Å | 1.279 Å; 1.404 Å | 1.385 Å (avg) |
| Ru-N (avg) | 2.065 Å | 2.056 Å | 2.058 Å |
| N-Ru-N (avg) (bite angle) | 78.20° | 78.82° | 78.60° |
| N-Ru-N (avg) (other angles) | 92.08° & 172.81° | 92.08° & 174.30° | 93.07° & 174.00° |
| Compound | Solvent | EA1/2 (V) | |||
|---|---|---|---|---|---|
| BPY reduction | Oxidation | ||||
| BPY0/−1 | BPY−1/−2 | BPY−2/−3 | Ru3+/2+ | ||
| 1 | CH3CN | −1.39 | −1.58 | −1.87 | +1.38 |
| 2 | CH3CN | −1.31 | −1.48 | −1.736 | +1.29 |
| 3 | CH3CN | −1.34 | +1.19 | ||
| ([Ru(bpy)3]2+) [11] | CH3CN | −1.31 | −1.50 | −1.77 | +1.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariappan, K.; Hussain, A.; Nisly, N.; Henning, T.J.; Goerl, K.A.; Alaparthi, M.; Sykes, A.G. Synthesis and X-ray Structures of Potential Light-Harvesting Ruthenium(II) Complexes. Molbank 2023, 2023, M1635. https://doi.org/10.3390/M1635
Mariappan K, Hussain A, Nisly N, Henning TJ, Goerl KA, Alaparthi M, Sykes AG. Synthesis and X-ray Structures of Potential Light-Harvesting Ruthenium(II) Complexes. Molbank. 2023; 2023(2):M1635. https://doi.org/10.3390/M1635
Chicago/Turabian StyleMariappan, Kadarkaraisamy, Anwar Hussain, Nathaniel Nisly, Tanner J. Henning, Kathryn A. Goerl, Madhubabu Alaparthi, and Andrew G. Sykes. 2023. "Synthesis and X-ray Structures of Potential Light-Harvesting Ruthenium(II) Complexes" Molbank 2023, no. 2: M1635. https://doi.org/10.3390/M1635
APA StyleMariappan, K., Hussain, A., Nisly, N., Henning, T. J., Goerl, K. A., Alaparthi, M., & Sykes, A. G. (2023). Synthesis and X-ray Structures of Potential Light-Harvesting Ruthenium(II) Complexes. Molbank, 2023(2), M1635. https://doi.org/10.3390/M1635






