Methyl 8- and 5-Bromo-1,4-Benzodioxane-2-carboxylate: Unambiguous Identification of the Two Regioisomers
Abstract
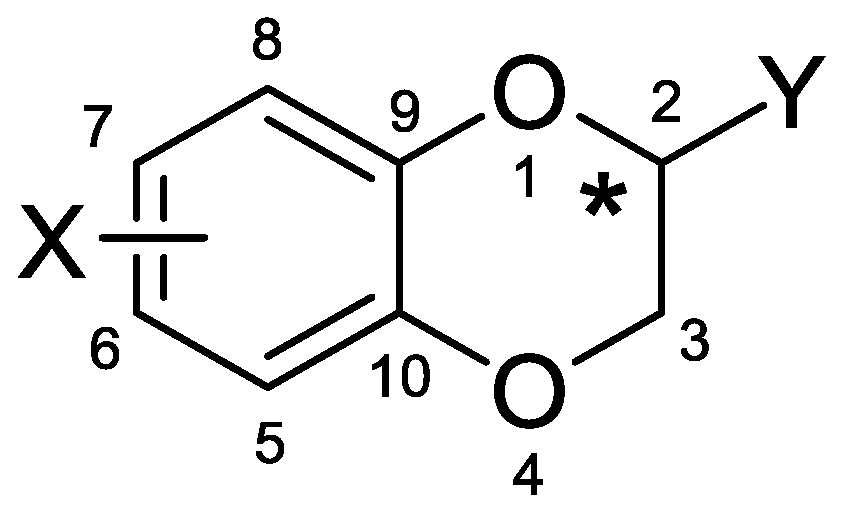
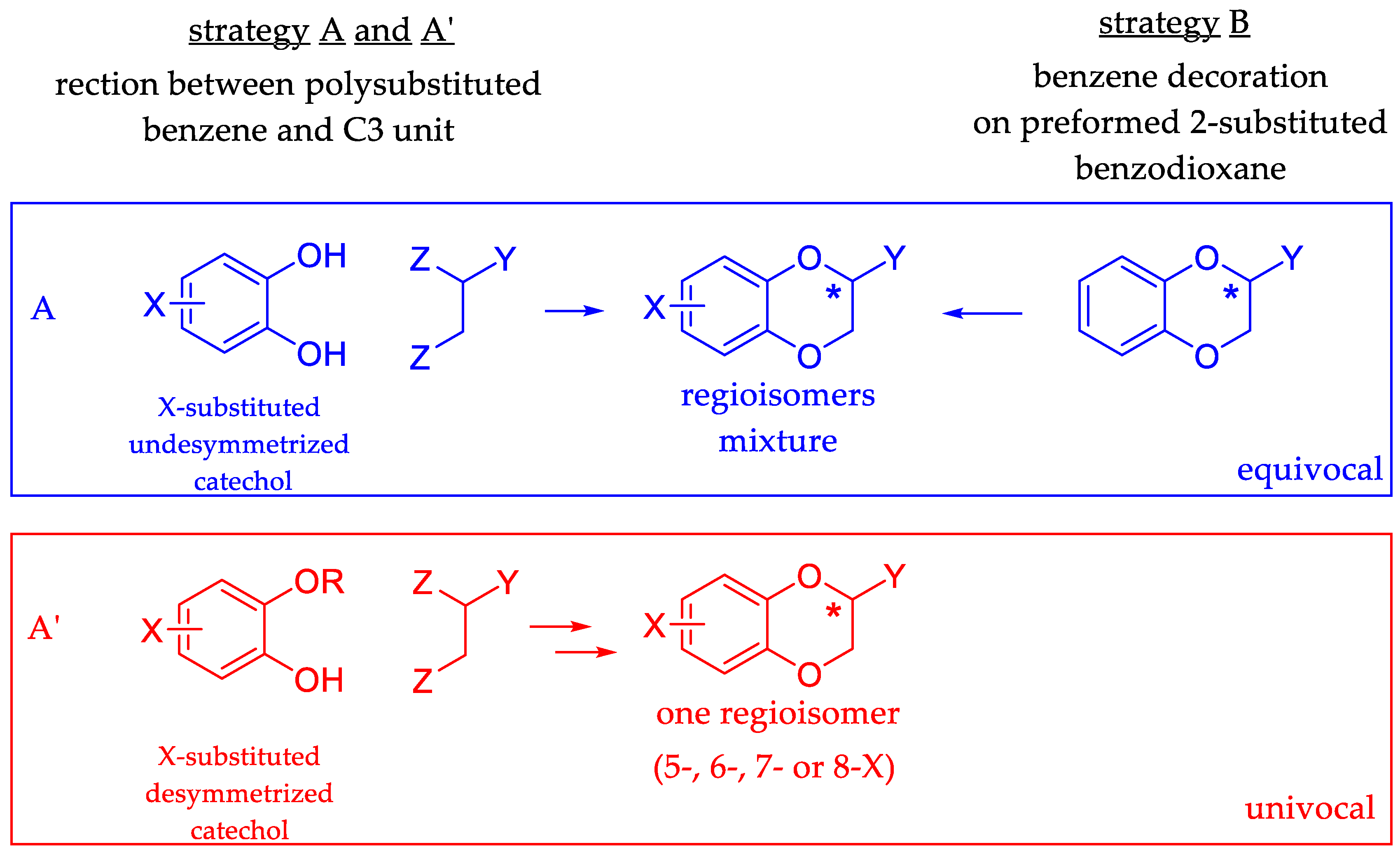
1. Introduction
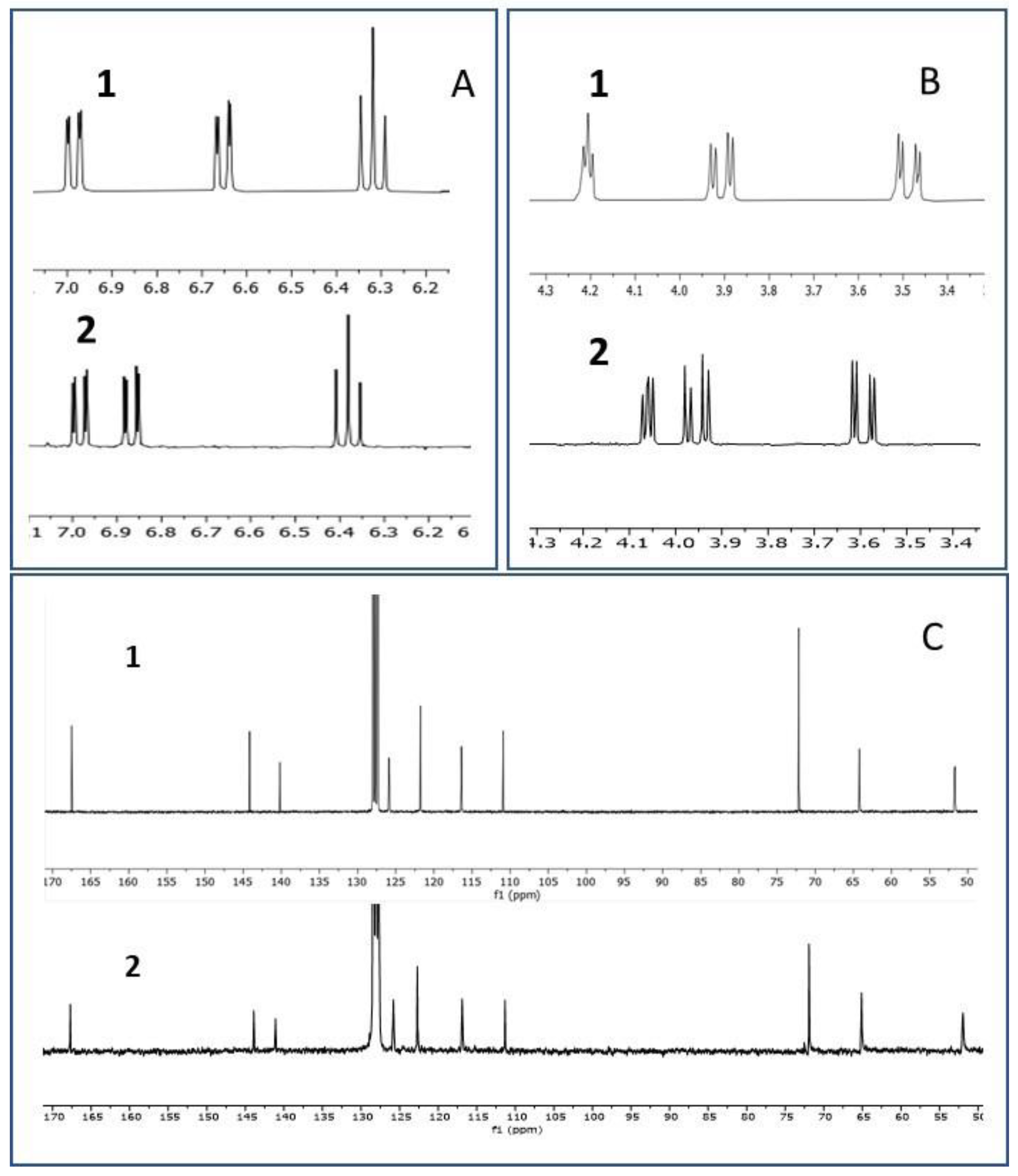
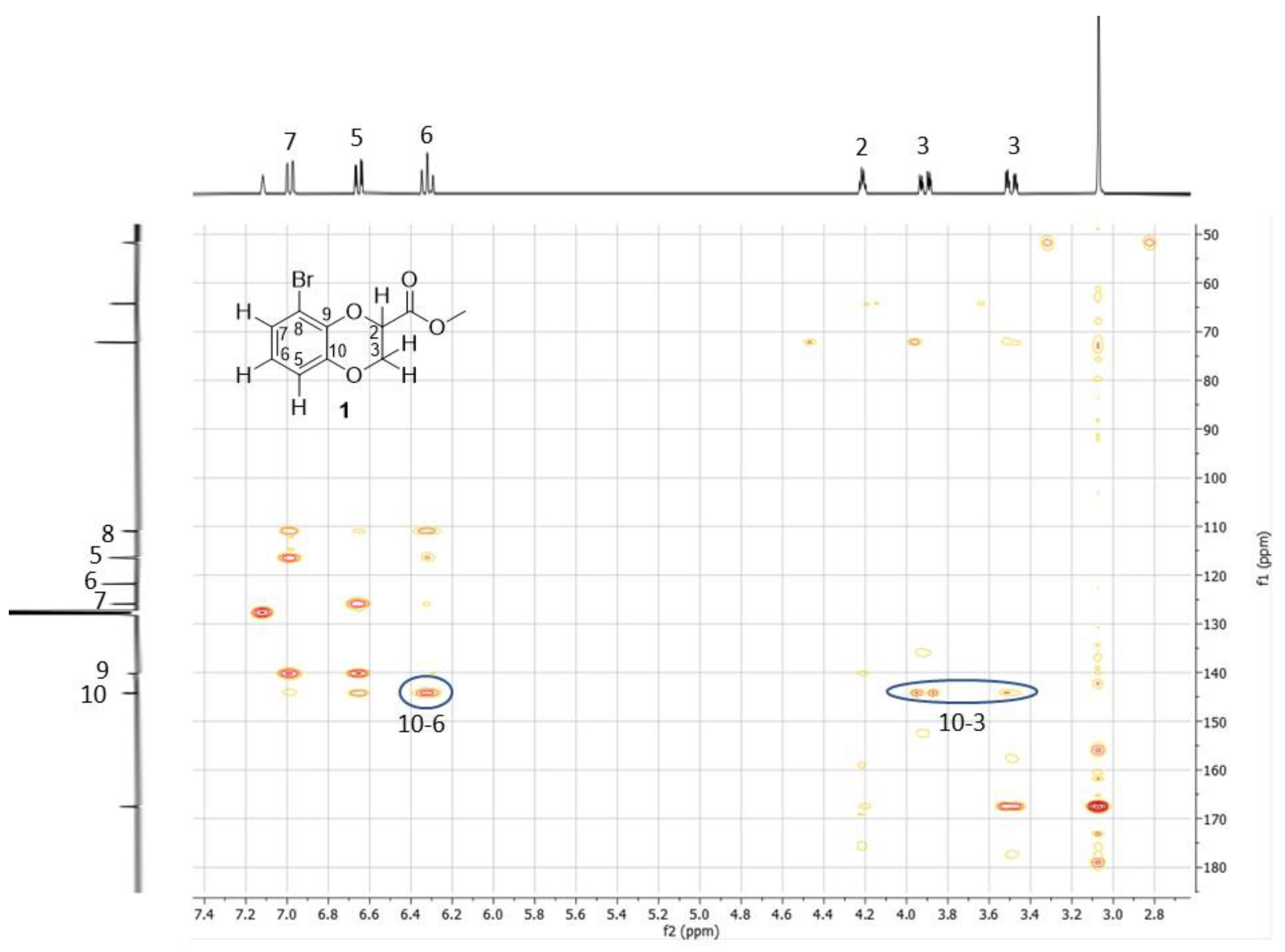
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bolchi, C.; Bavo, F.; Appiani, R.; Roda, G.; Pallavicini, M. 1,4-Benzodioxane, an evergreen, versatile scaffold in medicinal chemistry: A review of its recent applications in drug design. Eur. J. Med. Chem. 2020, 200, 112419. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, L.; Pallavicini, M.; Budriesi, R.; Gobbi, M.; Straniero, V.; Zagami, M.; Chiodini, G.; Bolchi, C.; Chiarini, A.; Micucci, M.; et al. Affinity and activity profiling of unichiral 8-substituited 1,4-benzodioxane analogues of WB4101 reveals a potent and selective α1B-adrenoceptor antagonist. Eur. J. Med. Chem. 2012, 58, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Bavo, F.; Pallavicini, M.; Gotti, C.; Appiani, R.; Moretti, M.; Colombo, S.F.; Pucci, S.; Viani, P.; Budriesi, R.; Renzi, M.; et al. Modifications at C(5) of 2-(2-pyrrolidinyl)-substituted 1,4-benzodioxane elicit potent α4β2 nicotinic acetylcholine receptor partial agonism with high selectivity over the α3β4 subtype. J. Med. Chem. 2020, 63, 15668–15692. [Google Scholar] [CrossRef] [PubMed]
- Bavo, F.; Pallavicini, M.; Appiani, R.; Bolchi, C. Determinants for α4β2 vs. α3β4 subtype selectivity of pyrrolidine-based nAChRs ligands: A computational perspective with focus on recent cryo-em receptor structures. Molecules 2021, 26, 3603. [Google Scholar] [CrossRef]
- Bolchi, C.; Valoti, E.; Straniero, V.; Ruggeri, P.; Pallavicini, M. From 2-aminomethyl-1,4-benzodioxane enantiomers to unichiral 2-cyano- and 2-carbonyl-substituted benzodioxanes via dichloroamine. J. Org. Chem. 2014, 79, 6732–6737. [Google Scholar] [CrossRef]
- Bouissane, L.; Khouili, M.; Coudert, G.; Pujol, M.D.; Guillaumet, G. New and promising type of leukotriene B4 (LTB4) antagonists based on the 1,4-benzodioxine structure. Eur. J. Med. Chem. 2023, 254, 115332. [Google Scholar] [CrossRef] [PubMed]
- Bolchi, C.; Bavo, F.; Pallavicini, M. Preparation and unequivocal identification of the regioisomers of nitrocatechol monobenzyl ether. Synth. Commun. 2017, 47, 1507–1513. [Google Scholar] [CrossRef]
- Bolchi, C.; Gotti, C.; Binda, M.; Fumagalli, L.; Pucci, L.; Pistillo, F.; Vistoli, G.; Valoti, E.; Pallavicini, M. Unichiral 2-(2′-pyrrolidinyl)-1,4-benzodioxanes: The 2R,2’S diastereomer of the N-methyl-7-hydroxy analogue is a potent α4β2 and α6β2-nicotinic acetylcholine receptor partial agonist. J. Med. Chem. 2011, 54, 7588–7601. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, G.; Pallavicini, M.; Zanotto, C.; Bissa, M.; Radaelli, A.; Straniero, V.; Bolchi, C.; Fumagalli, L.; Ruggeri, P.; De Morghen, C.G.; et al. Benzodioxane-benzamides as new bacterial cell division inhibitors. Eur. J. Med. Chem. 2015, 89, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Lalloz, L.; Loppinet, V. 2-Benzodioxinylaminoethanols: A new class of β-adrenergic blocking and antihypertensive agents. J. Med. Chem. 1981, 24, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Tan, C.W.; Chen, S.F.; Ku, H. One-pot neat reactions of carboxylic esters and alkylenediamines for efficient preparation of Nacylalkylenediamines. J. Org. Chem. 1998, 63, 10015–10017. [Google Scholar] [CrossRef]
- Sanchez, I.; Pujol, M.D.; Guillaumet, G.; Massingham, R.; Monteil, A.; Dureng, G.; Winslow, E. Design and synthesis of substituted compounds containing the 1,4-benzodioxin subunit. New potential calcium antagonists. Eur. J. Med. Chem. 2000, 35, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Peglion, J.L.; Goument, B.; Despaux, N.; Charlot, V.; Giraud, H.; Nisole, C.; Newman-Tancredi, A.; Dekeyne, A.; Bertrand, M.; Genissel, P.; et al. Improvement in the selectivity and metabolic stability of the serotonin 5-HT1A ligand, S 15535: A series of cis- and trans-2-(arylcycloalkylamine) 1-indanols. J. Med. Chem. 2002, 45, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; McCorvy, J.D.; Fischer, M.G.; Butler, K.V.; Shen, Y.; Roth, B.L.; Jn, J. Discovery of G protein-biased D2 dopamine receptor partial agonists. J. Med. Chem. 2016, 59, 10601–10618. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armano, E.; Giraudo, A.; Pallavicini, M.; Bolchi, C. Methyl 8- and 5-Bromo-1,4-Benzodioxane-2-carboxylate: Unambiguous Identification of the Two Regioisomers. Molbank 2023, 2023, M1623. https://doi.org/10.3390/M1623
Armano E, Giraudo A, Pallavicini M, Bolchi C. Methyl 8- and 5-Bromo-1,4-Benzodioxane-2-carboxylate: Unambiguous Identification of the Two Regioisomers. Molbank. 2023; 2023(2):M1623. https://doi.org/10.3390/M1623
Chicago/Turabian StyleArmano, Edoardo, Alessandro Giraudo, Marco Pallavicini, and Cristiano Bolchi. 2023. "Methyl 8- and 5-Bromo-1,4-Benzodioxane-2-carboxylate: Unambiguous Identification of the Two Regioisomers" Molbank 2023, no. 2: M1623. https://doi.org/10.3390/M1623
APA StyleArmano, E., Giraudo, A., Pallavicini, M., & Bolchi, C. (2023). Methyl 8- and 5-Bromo-1,4-Benzodioxane-2-carboxylate: Unambiguous Identification of the Two Regioisomers. Molbank, 2023(2), M1623. https://doi.org/10.3390/M1623






