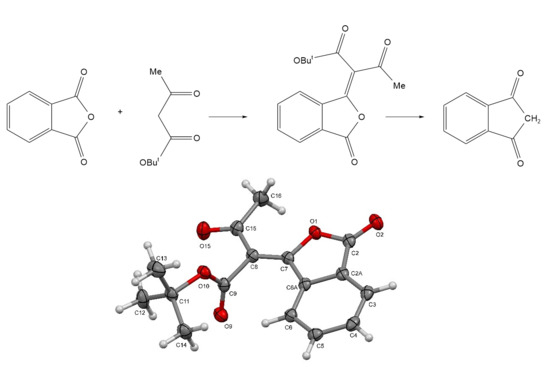
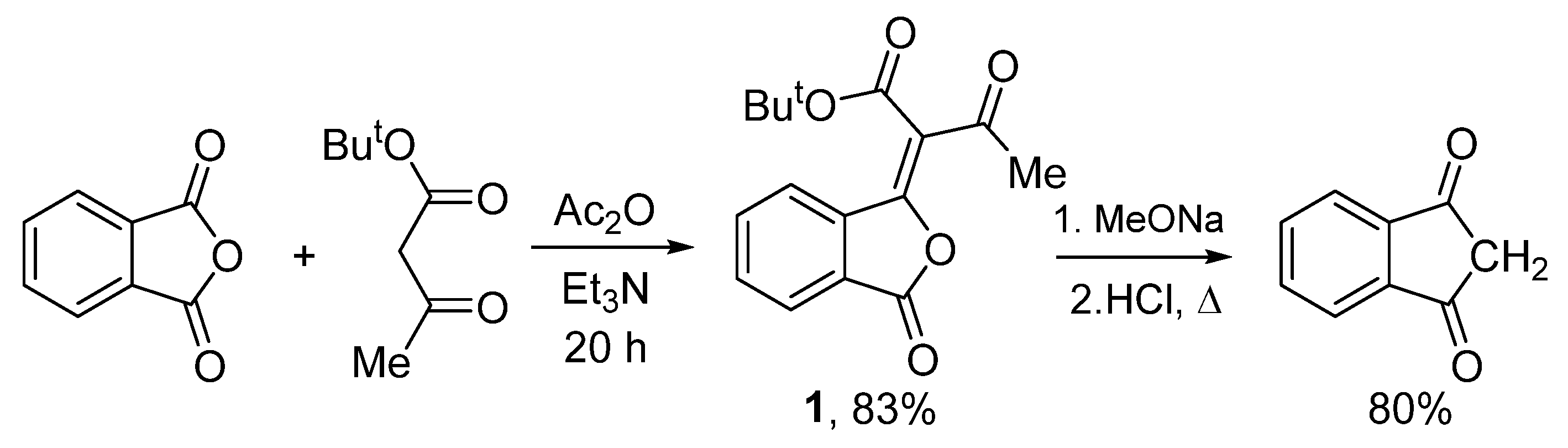
tert-Butyl (E)-3-oxo-2-(3-oxoisobenzofuran-1(3H)-ylidene)butanoate
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Ma, R.; Liu, T.; Yu, J.; Xiao, Y.; Sun, R.; Xie, G.; Yuan, J.; Chen, Y.; Chen, K.; et al. Fine-Tuning Energy Levels via Asymmetric End Groups Enables Polymer Solar Cells with Efficiencies over 17%. Joule 2020, 4, 1236–1247. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Li, C.; Zheng, N.; Xie, Z.; Ma, Z.; Lu, Y.; Wan, X.; Chen, Y. Effect of Nitro-Substituted Ending Groups on the Photovoltaic Properties of Nonfullerene Acceptors. ACS Appl. Mater. Interfaces 2020, 12, 41861–41868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, J.; Xu, Z.; Xu, H.; Quan, J.; Deng, J.; Li, Y.; Tong, Y.; Hu, B.; Chen, L. Recent advances of nonfullerene acceptors in organic solar cells. Nano Energy 2022, 103, 107802. [Google Scholar] [CrossRef]
- Luo, D.; Jang, W.; Babu, D.D.; Kim, M.S.; Wang, D.H.; Kyaw, A.K.K. Recent progress in organic solar cells based on non-fullerene acceptors: Materials to devices. J. Mater. Chem. A 2022, 10, 3255–3295. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Qiu, N.; Feng, H.; Gao, H.; Kan, B.; Ma, Y.; Li, C.; Wan, X.; Chen, Y. A Halogenation Strategy for over 12% Efficiency Nonfullerene Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1702870. [Google Scholar] [CrossRef]
- He, C.; Chen, Z.; Wang, T.; Shen, Z.; Li, Y.; Zhou, J.; Yu, J.; Fang, H.; Li, Y.; Li, S.; et al. Asymmetric electron acceptor enables highly luminescent organic solar cells with certified efficiency over 18%. Nat. Commun. 2022, 13, 2598. [Google Scholar] [CrossRef]
- Fu, H.; Yao, J.; Zhang, M.; Xue, L.; Zhou, Q.; Li, S.; Lei, M.; Meng, L.; Zhang, Z.-G.; Li, Y. Low-cost synthesis of small molecule acceptors makes polymer solar cells commercially viable. Nat. Commun. 2022, 13, 3687. [Google Scholar] [CrossRef]
- Planells, M.; Robertson, N. Naphthyl Derivatives Functionalised with Electron Acceptor Units—Synthesis, Electronic Characterisation and DFT Calculations. Eur. J. Org. Chem. 2012, 2012, 4947–4953. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, R.; Meng, Q.; Zhang, X.; Sun, Y. Discovery of new protein kinase CK2 inhibitors with 1,3-dioxo-2,3-dihydro-1H-indene core. Medchemcomm 2016, 7, 1352–1355. [Google Scholar] [CrossRef]
- Aldrich, T.J.; Matta, M.; Zhu, W.; Swick, S.M.; Stern, C.L.; Schatz, G.C.; Facchetti, A.; Melkonyan, F.S.; Marks, T.J. Fluorination Effects on Indacenodithienothiophene Acceptor Packing and Electronic Structure, End-Group Redistribution, and Solar Cell Photovoltaic Response. J. Am. Chem. Soc. 2019, 141, 3274–3287. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Zhang, J.; Zhang, G.; Liu, C.; Tang, H.; Zhang, K.; Huang, F. Rationally regulating the terminal unit and copolymerization spacer of polymerized small-molecule acceptors for all-polymer solar cells with high open-circuit voltage over 1.10 V. J. Mater. Chem. A 2022, 10, 15932–15940. [Google Scholar] [CrossRef]
- Li, M.; Xiong, Q.; Qu, B.; Xiao, Y.; Lan, Y.; Lu, L.; Xiao, W. Utilizing Vinylcyclopropane Reactivity: Palladium-Catalyzed Asymmetric [5+2] Dipolar Cycloadditions. Angew. Chem. Int. Ed. 2020, 59, 17429–17434. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Igci, C.; Yang, Y.; Syzgantseva, O.A.; Syzgantseva, M.A.; Rakstys, K.; Kanda, H.; Shibayama, N.; Ding, B.; Zhang, X.; et al. Dopant-Free Hole Transport Materials Afford Efficient and Stable Inorganic Perovskite Solar Cells and Modules. Angew. Chem. Int. Ed. 2021, 60, 20489–20497. [Google Scholar] [CrossRef]
- Yu, H.; Luo, S.; Sun, R.; Angunawela, I.; Qi, Z.; Peng, Z.; Zhou, W.; Han, H.; Wei, R.; Pan, M.; et al. A Difluoro-Monobromo End Group Enables High-Performance Polymer Acceptor and Efficient All-Polymer Solar Cells Processable with Green Solvent under Ambient Condition. Adv. Funct. Mater. 2021, 31, 2100791. [Google Scholar] [CrossRef]
- Mkrtchyan, S.; Chilingaryan, Z.; Ghazaryan, G.; Dede, R.; Rasool, N.; Rashid, M.; Villinger, A.; Görls, H.; Karapetyan, G.; Ghochikyan, T.; et al. E/Z-Selective Synthesis of Alkylidene-3-oxo-3H-isobenzofurans by Reaction of Silyl Enol Ethers with Phthaloyl Dichloride. Synthesis 2011, 2011, 2281–2290. [Google Scholar] [CrossRef]
- CrysAlisPro. Version 1.171.41.106a. In Rigaku Oxford Diffraction; Rigaku Corporation: Oxford, UK, 2021. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 229–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Kaliyaperumal Appaye, S.; Pandurang Nikumbh, S.; Reddy Govindapur, R.; Banerjee, S.; Bhalerao, D.S.; Syam Kumar, U.K. Ethenolate Transfer Reactions: A Facile Synthesis of Vinyl Esters. Helv. Chim. Acta 2014, 97, 1115–1122. [Google Scholar] [CrossRef]
- He, G.; Wu, C.; Zhou, J.; Yang, Q.; Zhang, C.; Zhou, Y.; Zhang, H.; Liu, H. A Method for Synthesis of 3-Hydroxy-1-indanones via Cu-Catalyzed Intramolecular Annulation Reactions. J. Org. Chem. 2018, 83, 13356–13362. [Google Scholar] [CrossRef] [PubMed]

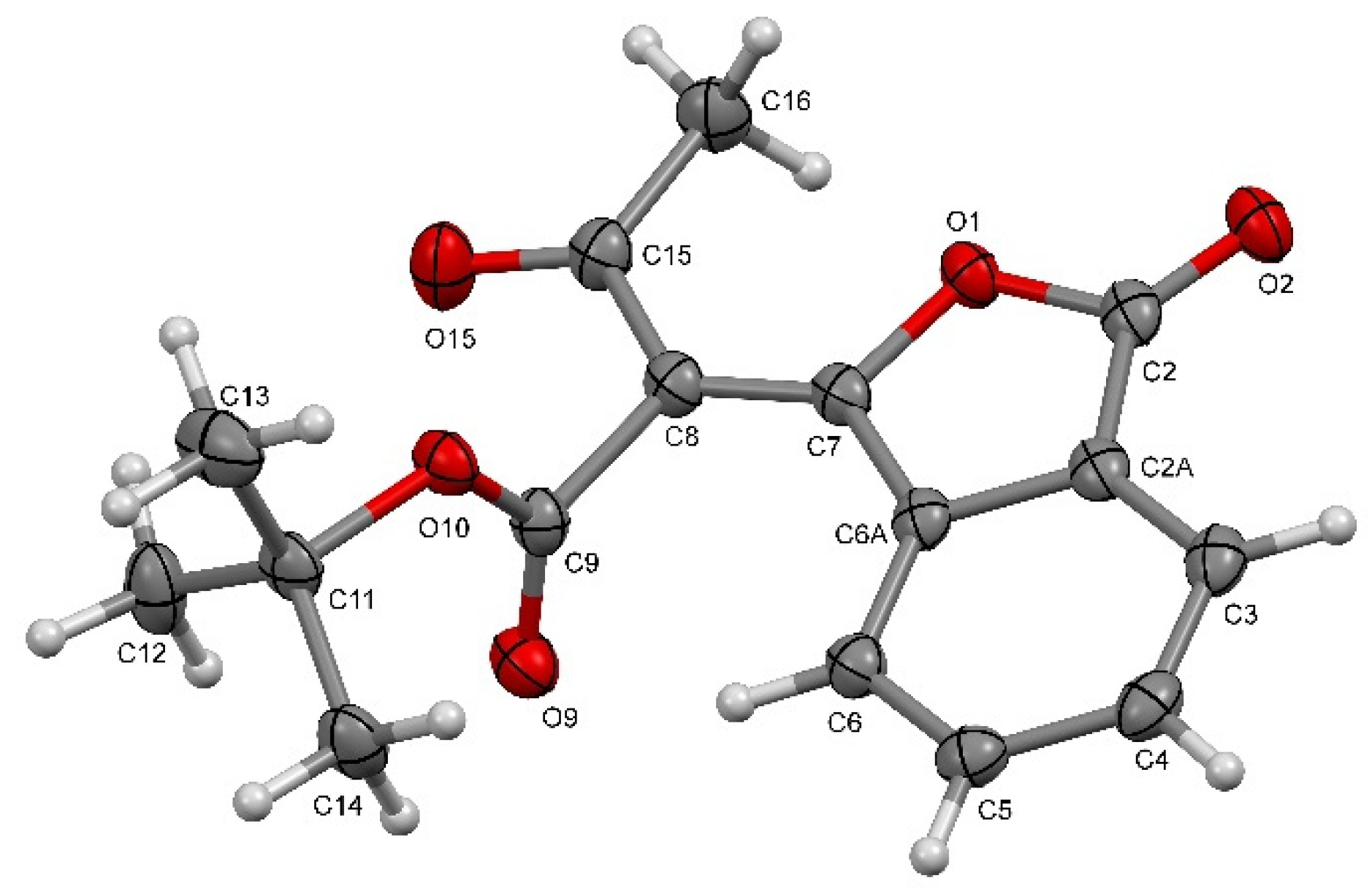
| Empirical Formula | C16H16O5 |
|---|---|
| Formula weight | 288.29 |
| Temperature | 99.9(2) K |
| Wavelength | 1.54184 Å |
| Crystal system | Monoclinic |
| Space group | P 21/c |
| Unit cell dimensions | a = 6.68587(5) Å a = 90° b = 10.54420(10) Å b = 94.2747(7)° c = 20.48957(18) Å g = 90° |
| Volume | 1440.44(2) Å3 |
| Z | 4 |
| Density (calculated) | 1.329 g/cm3 |
| Absorption coefficient | 0.824 mm−1 |
| F(000) | 608 |
| Crystal size | 0.4 × 0.16 × 0.03 mm3 |
| Theta range for data collection | 4.328 to 77.931°. |
| Index ranges | −7 <= h <= 8, −13 <= k <= 13, −25 <= l <= 25 |
| Reflections collected | 16,388 |
| Independent reflections | 3062 [R(int) = 0.0234] |
| Observed reflections | 2878 |
| Completeness to theta = 67.684° | 100.0% |
| Absorption correction | Gaussian |
| Max. and min. transmission | 1.000 and 0.434 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3062/0/194 |
| Goodness-of-fit on F2 | 1.055 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0338, wR2 = 0.0874 |
| R indices (all data) | R1 = 0.0356, wR2 = 0.0890 |
| Largest diff. peak and hole | 0.194 and −0.230 e.Å−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chechulina, A.S.; Knyazeva, E.A.; Kan, B.; Duan, T.; Rakitin, O.A. tert-Butyl (E)-3-oxo-2-(3-oxoisobenzofuran-1(3H)-ylidene)butanoate. Molbank 2023, 2023, M1614. https://doi.org/10.3390/M1614
Chechulina AS, Knyazeva EA, Kan B, Duan T, Rakitin OA. tert-Butyl (E)-3-oxo-2-(3-oxoisobenzofuran-1(3H)-ylidene)butanoate. Molbank. 2023; 2023(2):M1614. https://doi.org/10.3390/M1614
Chicago/Turabian StyleChechulina, Alexandra S., Ekaterina A. Knyazeva, Bin Kan, Tainan Duan, and Oleg A. Rakitin. 2023. "tert-Butyl (E)-3-oxo-2-(3-oxoisobenzofuran-1(3H)-ylidene)butanoate" Molbank 2023, no. 2: M1614. https://doi.org/10.3390/M1614
APA StyleChechulina, A. S., Knyazeva, E. A., Kan, B., Duan, T., & Rakitin, O. A. (2023). tert-Butyl (E)-3-oxo-2-(3-oxoisobenzofuran-1(3H)-ylidene)butanoate. Molbank, 2023(2), M1614. https://doi.org/10.3390/M1614