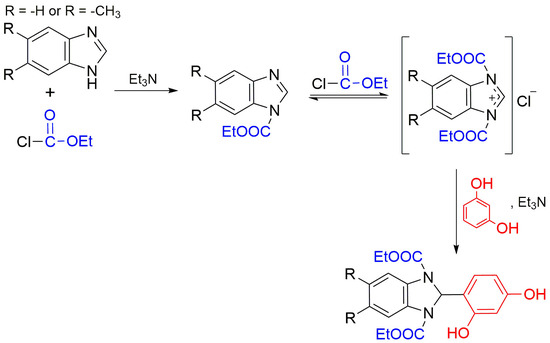
New 2-(2,4-Dihydroxyphenyl)benzimidazolines
Abstract
1. Introduction
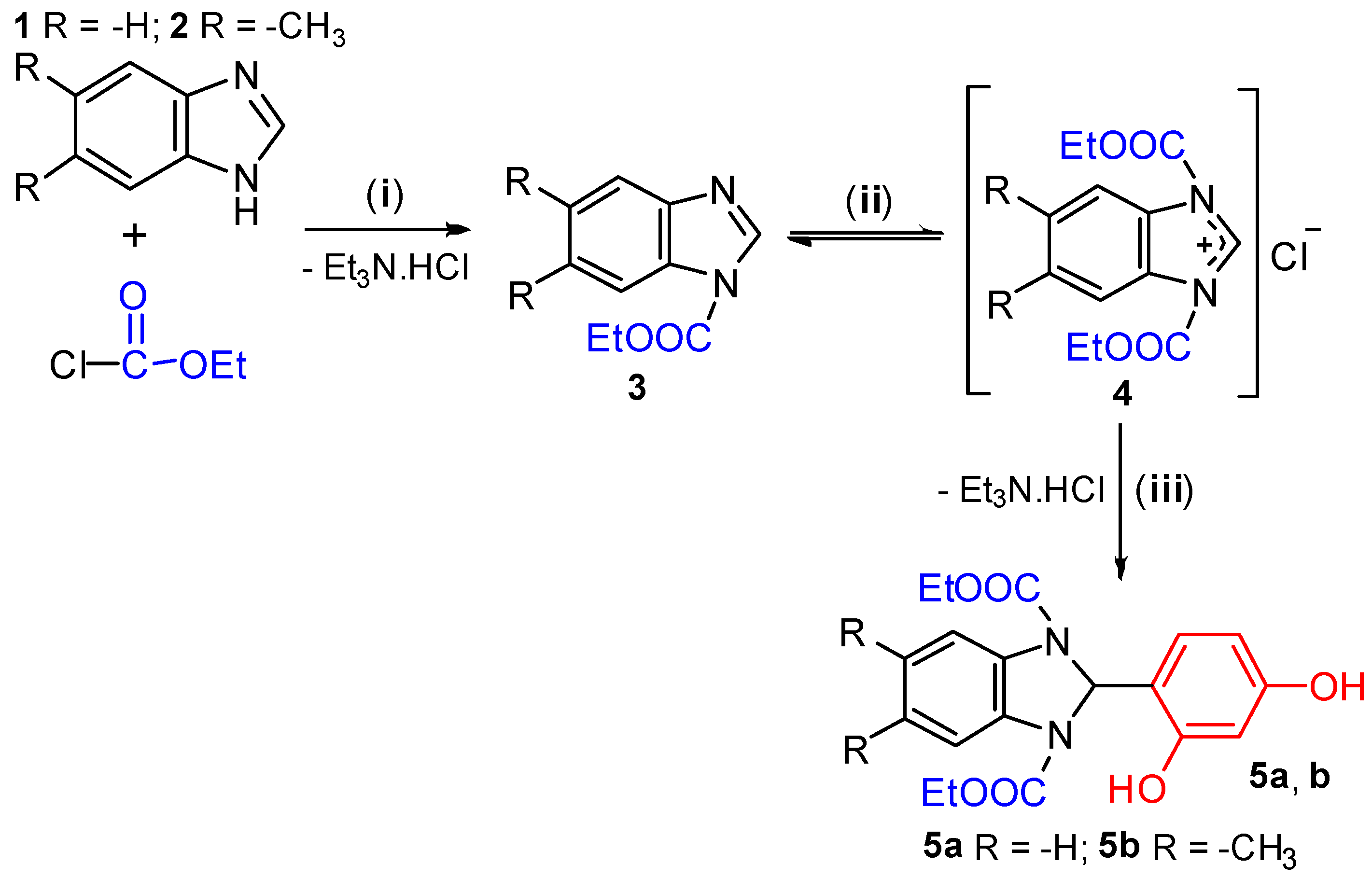
2. Results and Discussion
3. Materials and Methods
Synthesis of 2-(2,4-Dihydroxyphenyl)benzimidazolines (5a,b), General Procedure
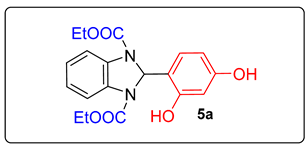
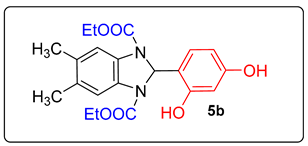
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karthikeyan, C.; Solomon, V.R.; Lee, H.; Trivedi, P. Synthesis and biological evaluation of 2-(phenyl)-3H-benzo[d]imidazole-5-carboxylic acids and its methyl esters as potent anti-breast cancer agents. Arab. J. Chem. 2017, 10, 1788–1794. [Google Scholar] [CrossRef]
- Gulcan, H.O.; Mavideniz, A.; Sahin, M.F.; Orhan, I.E. Benzimidazole-derived compounds designed for different targets of Alzheimer’s disease. Curr. Med. Chem. 2019, 26, 3260–3278. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine. Acta Pharm. Sin. B 2022, 13, 478–497. [Google Scholar] [CrossRef] [PubMed]
- Bino, A.; Baldisserotto, A.; Scalambra, E.; Dissette, V.; Vedaldi, D.E.; Salvador, A.; Durini, E.; Manfredini, S.; Vertuani, S. Design, synthesis and biological evaluation of novel hydroxy-phenyl-1H-benzimidazoles as radical scavengers and UV-protective agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J.; Skrzypek, A.; Karpinska, M.; Czarnecka, K.; Szymanski, P.; Bajda, M.; Niewiadomy, A. Biological evaluation, molecular docking, and SAR studies of novel 2-(2,4-dihydroxyphenyl)-1H-benzimidazole analogues. Biomolecules 2019, 9, 870. [Google Scholar] [CrossRef]
- Padalkar, V.S.; Borse, B.N.; Gupta, V.D.; Phatangare, K.R.; Patil, V.S.; Umape, P.G.; Sekar, N. Synthesis and antimicrobial activity of novel 2-substituted benzimidazole, benzoxazole and benzothiazole derivatives. Arab. J. Chem. 2016, 9, 1125–1130. [Google Scholar] [CrossRef]
- Ismail, M.A.; Brun, R.; Wenzler, T.; Tanious, F.A.; Wilson, W.D.; Boykin, D.W. Dicationic biphenyl benzimidazole derivatives as antiprotozoal agents. Bioorg. Med. Chem. 2004, 12, 5405–5413. [Google Scholar] [CrossRef]
- Racané, L.; Cindrić, M.; Perin, N.; Roškarić, P.; Starčević, K.; Mašek, T.; Maurić, M.; Dogan, J.; Karminski-Zamola, G. Synthesis and antioxidative potency of novel amidino substituted benzimidazole and benzothiazole derivatives. Croat. Chem. Acta 2017, 90, 187–195. [Google Scholar] [CrossRef]
- Karpińka, M.M.; Matysiak, J.; Niewiadomy, A. Synthesis of novel 4-(1H-benzimidazol-2-yl)benzene-1,3-diols and their cytotoxic activity against human cancer cell lines. Arch. Pharm. Res. 2011, 34, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Gellis, A.; Kovacic, H.; Boufatah, N.; Vanelle, P. Synthesis and cytotoxicity evaluation of some benzimidazole-4,7-diones as bioreductive anticancer agents. Eur. J. Med. Chem. 2008, 43, 1858–1864. [Google Scholar] [CrossRef]
- Matysiak, J.; Opolski, A. Synthesis and antiproliferative activity of N-substituted 2-amino-5-(2,4-dihydroxyphenyl)-1,3,4-thia-diazoles. Bioorg. Med. Chem. 2006, 14, 4483–4489. [Google Scholar] [CrossRef]
- Kiprof, P.; Carlson, J.C.; Anderson, D.R.; Nemykin, V.N. Systematic color tuning of a family of luminescent azole-based organoboron compounds suitable for OLED applications. Dalton Trans. 2013, 42, 15120–15132. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Padalkar, V.S.; Tathe, A.B.; Gupta, V.D.; Sekar, N. Synthesis, photo-physical and DFT studies of ESIPT inspired novel 2-(2′,4′-dihydroxyphenyl) benzimidazole, benzoxazole and benzothiazole. J. Fluoresc. 2013, 23, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Padalkar, V.S.; Tathe, A.; Gupta, V.D.; Patil, V.S.; Phatangare, K.; Sekar, N. Synthesis and photo-physical characteristics of ESIPT inspired 2-substituted benzimidazole, benzoxazole and benzothiazole fluorescent derivatives. J. Fluoresc. 2012, 22, 311–322. [Google Scholar] [CrossRef]
- Roush, W.R. Total synthesis of biologically active natural products. J. Am. Chem. Soc. 2008, 130, 6654–6656. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds—Biological Activity; InTech: London, UK, 2017; Chapter 1. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: Nottingham, UK, 2009; p. 53. [Google Scholar]
- Alihosseini, F. Plant-based compounds for antimicrobial textiles. In Antimicrobial Textiles; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 155–195. [Google Scholar] [CrossRef]
- Khan, K.M.; Mesaik, M.A.; Abdalla, O.M.; Rahim, F.; Soomro, S.; Halim, S.A.; Mustafa, G.; Ambreen, N.; Khalid, A.S.; Taha, M.; et al. The immunomodulation potential of the synthetic derivatives of benzothiazoles: Implications in immune system disorders through in vitro and in silico studies. Bioorg. Chem. 2016, 64, 21–28. [Google Scholar] [CrossRef]
- Chari, M.A.; Shobha, D.; Sasaki, T. Room temperature synthesis of benzimidazole derivatives using reusable cobalt hydroxide (II) and cobalt oxide (II) as efficient solid catalysts. Tetrahedron Lett. 2011, 52, 5575–5580. [Google Scholar] [CrossRef]
- Han, F.; Zhang, X.; Zhang, J.; Wei, Y.; Zhang, X.; Huang, W.; Xu, H. 3D-Encapsulated iridium-complexed nanophosphors for highly efficient host-free organic light-emitting diodes. Chem. Commun. 2016, 52, 5183–5186. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Khan, M.; Ambreen, N.; Rahim, F.; Naureen, S.; Perveen, S.; Choudhary, M.L.; Voelter, W. Synthesis and β-Glucuronidase Inhibitory Potential of Benzimidazole Derivatives. J. Med. Chem. 2012, 8, 421–427. [Google Scholar] [CrossRef]
- Castillo-García, A.A.; González-Sebastián, L.; Lomas-Romero, L.; Hernandez-Ortega, S.; Toscano, R.A.; Morales-Morales, D. Novel meta-benzothiazole and benzimidazole functionalised POCOP-Ni(ii) pincer complexes as efficient catalysts in the production of diarylketones. New J. Chem. 2021, 45, 10204–10216. [Google Scholar] [CrossRef]
- Shi, Y.; Meng, X.; Yang, H.; Song, L.; Liu, S.; Xu, A.; Chen, Z.; Huang, W.; Zhao, Q. Lysosome-specific sensing and imaging of pH variations in vitro and in vivo utilizing a near-infrared boron complex. J. Mater. Chem. B 2019, 7, 3569–3575. [Google Scholar] [CrossRef]
- Mazurkiewicz, R.; Październiok-Holewa, A.; Adamek, J.; Zielińska, K. α-Amidoalkylating agents: Structure, synthesis, reactivity and application. Adv. Heterocycl. Chem. 2014, 111, 43–93. [Google Scholar] [CrossRef]
- Dondoni, A.; Dall’Occo, T.; Galliani, G.; Mastellari, A.; Medici, A. Addition of 2-silylazoles to heteroaryl cations. Synthesis of unsymmetrical azadiaryls. Tetrahedron Lett. 1984, 25, 3637–3640. [Google Scholar] [CrossRef]
- Itoh, T.; Hasegawa, H.; Nagata, K.; Ohsawa, A. Allylation of Azoles with Allyltributyltin via Unstable N-(Alkoxycarbonyl)azlium Salts. J. Org. Chem. 1994, 59, 1319–1325. [Google Scholar] [CrossRef]
- Itoh, T.; Miyazaki, M.; Nagata, K.; Ohsawa, A. Addition Reaction of Imidazoles and Thiazoles with Silyl Enol Ethers in the Presence of Alkyl Chloroformate. Tetrahedron Lett. 2000, 56, 4383–4395. [Google Scholar] [CrossRef]
- Beveridge, R.E.; Black, D.A.; Arndtsen, B.A. Copper-Catalyzed multicomponent coupling of organoindium reagents with nitrogen-containing aromatic heterocycles. Eur. J. Org. Chem. 2010, 19, 3650–3656. [Google Scholar] [CrossRef]
- Stremski, Y.; Kirkova, D.; Statkova-Abeghe, S.; Angelov, P.; Ivanov, I.; Georgiev, D. Synthesis and antibacterial activity of hydroxylated 2-arylbenzothiazole derivatives. Synth. Commun. 2020, 50, 3007–3015. [Google Scholar] [CrossRef]
- Kirkova, D.; Stremski, Y.; Statkova-Abeghe, S.; Docheva, M. Quercetin Hybrids-Synthesis, Spectral Characterization and Radical Scavenging Potential. Molbank 2022, 2022, M1329. [Google Scholar] [CrossRef]
| Product 5 | R | Ratio * | Time, h | Yield, % |
|---|---|---|---|---|
| a | H | 1:1 | 24 | 50 |
| a | H | 1:2 | 5 | 89 |
| b | Me | 1:1 | 24 | 56 |
| b | Me | 1:2 | 5 | 92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stremski, Y.; Bachvarova, M.; Kirkova, D.; Statkova-Abeghe, S. New 2-(2,4-Dihydroxyphenyl)benzimidazolines. Molbank 2023, 2023, M1602. https://doi.org/10.3390/M1602
Stremski Y, Bachvarova M, Kirkova D, Statkova-Abeghe S. New 2-(2,4-Dihydroxyphenyl)benzimidazolines. Molbank. 2023; 2023(1):M1602. https://doi.org/10.3390/M1602
Chicago/Turabian StyleStremski, Yordan, Maria Bachvarova, Desislava Kirkova, and Stela Statkova-Abeghe. 2023. "New 2-(2,4-Dihydroxyphenyl)benzimidazolines" Molbank 2023, no. 1: M1602. https://doi.org/10.3390/M1602
APA StyleStremski, Y., Bachvarova, M., Kirkova, D., & Statkova-Abeghe, S. (2023). New 2-(2,4-Dihydroxyphenyl)benzimidazolines. Molbank, 2023(1), M1602. https://doi.org/10.3390/M1602