6,6′-Di-(8″-quinoline)-2,2′-bipyridine Cobalt(II) Complex
Abstract
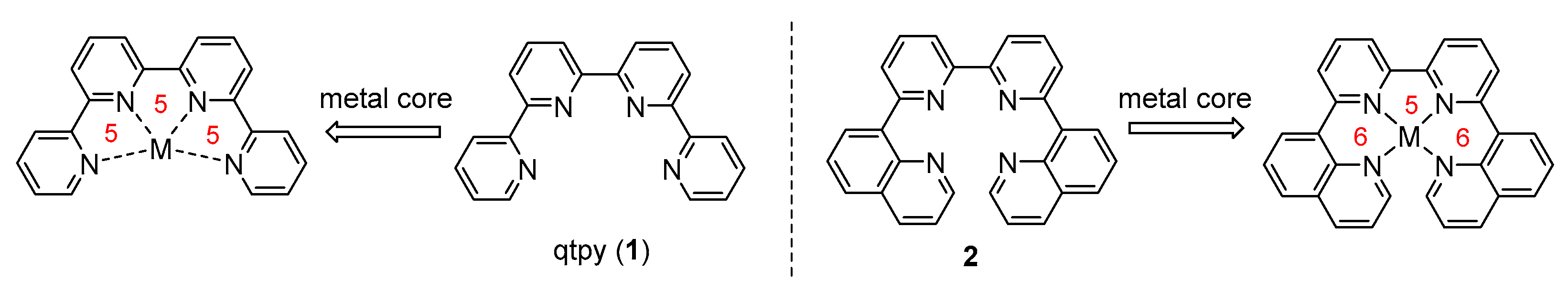
1. Introduction
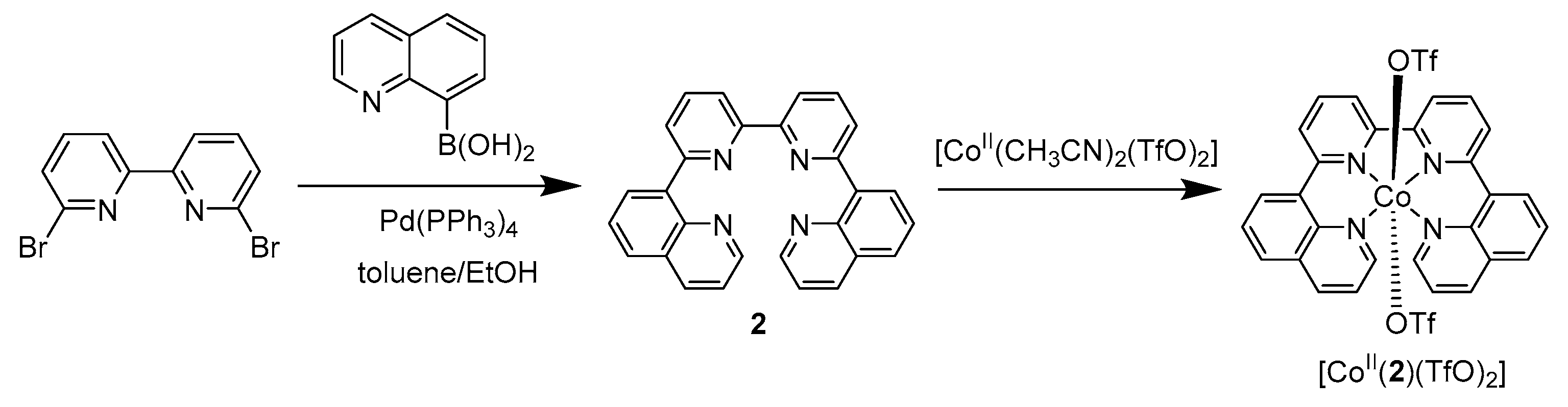
2. Results and Discussion
3. Materials and Methods
- Synthesis of 6,6′-di-(8″-quinoline)-2,2′-bipyridine (2).
- Synthesis of [CoII(2)(TfO)2].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gorczynski, A.; Harrowfield, J.M.; Patroniak, V.; Stefankiewicz, A.R. Quaterpyridines as Scaffolds for Functional Metallosupramolecular Materials. Chem. Rev. 2016, 116, 14620–14674. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Duan, L.; Zhou, A.; Thummel, R.P. First-row transition metal polypyridine complexes that catalyze proton to hydrogen reduction. Coord. Chem. Rev. 2020, 402, 213079. [Google Scholar] [CrossRef]
- Tong, L.; Thummel, R.P. Mononuclear ruthenium polypyridine complexes that catalyze water oxidation. Chem. Sci. 2016, 7, 6591–6603. [Google Scholar] [CrossRef] [PubMed]
- Renouard, T.; Fallahpour, R.A.; Nazeeruddin, M.K.; Humphry-Baker, R.; Gorelsky, S.I.; Lever, A.B.P.; Grätzel, M. Novel Ruthenium Sensitizers Containing Functionalized Hybrid Tetradentate Ligands: Synthesis, Characterization, and INDO/S Analysis. Inorg. Chem. 2002, 41, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Barolo, C.; Nazeeruddin, M.K.; Fantacci, S.; Di Censo, D.; Comte, P.; Liska, P.; Viscardi, G.; Quagliotto, P.; De Angelis, F.; Ito, S.; et al. Synthesis, characterization, and DFT-TDDFT computational study of a ruthenium complex containing a functionalized tetradentate ligand. Inorg. Chem. 2006, 45, 4642–4653. [Google Scholar] [CrossRef]
- Liu, Y.; Ng, S.-M.; Yiu, S.-M.; Lam, W.W.Y.; Wei, X.-G.; Lau, K.-C.; Lau, T.-C. Catalytic Water Oxidation by Ruthenium(II) Quaterpyridine (qpy) Complexes: Evidence for Ruthenium(III) qpy-N,N‴-dioxide as the Real Catalysts. Angew. Chem. Int. Ed. 2014, 53, 14468–14471. [Google Scholar] [CrossRef]
- Chan, C.-W.; Lai, T.-F.; Che, C.-M. Electrochemical oxidation of diaquaruthenium(II) complexes of quaterpyridines and crystal structure of [RuL1(PPh3)2][ClO4]2(L1= 3″,5,5′,5‴-tetramethyl-2,2′: 6′,2″: 6″,2‴-quaterpyridine). J. Chem. Soc. Dalton Trans. 1994, 6, 895–899. [Google Scholar] [CrossRef]
- Dell’Amico, D.B.; Calderazzo, F.; Englert, U.; Labella, L.; Marchetti, F. The first crystallographically established bis-qtpy (qtpy = 2,2′:6′,2″:6″,2‴-quaterpyridine) metal complex. J. Chem. Soc. Dalton Trans. 2001, 357–358. [Google Scholar] [CrossRef]
- Belli Dell’ Amico, D.; Calderazzo, F.; Curiardi, M.; Labella, L.; Marchetti, F. Bis-qtpy (qtpy = 2,2′:6′,2″:6″,2‴-quaterpyridine) Metal Complexes, [M(qtpy)2]2+. Inorg. Chem. 2004, 43, 5459–5465. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Hannon, M.J.; Martin, A.; Raithby, P.R.; Tocher, D.A. Self-assembly of double-helical complexes of 2,2′:6′,2″:6″,2‴-Quaterpyridine (qtpy); The x-ray crystal structures of [Cu2(qtpy)2][PF6]2 and [Ag2(qtpy)2][BF4]2. Polyhedron 1992, 11, 2967–2971. [Google Scholar] [CrossRef]
- Tong, L.; Zong, R.; Thummel, R.P. Visible light-driven hydrogen evolution from water catalyzed by a molecular cobalt complex. J. Am. Chem. Soc. 2014, 136, 4881–4884. [Google Scholar] [CrossRef] [PubMed]
- Maslen, E.N.; Raston, C.L.; White, A.H. Crystal structure of aqua(2,2′:6′,2″:6″,2‴-quaterpyridyl)sulphitocobalt-(III) nitrate monohydrate. J. Chem. Soc. Dalton Trans. 1975, 323–326. [Google Scholar] [CrossRef]
- Liu, J.; Liao, R.Z.; Heinemann, F.W.; Meyer, K.; Thummel, R.P.; Zhang, Y.; Tong, L. Electrocatalytic Hydrogen Evolution by Cobalt Complexes with a Redox Non-Innocent Polypyridine Ligand. Inorg. Chem. 2021, 60, 17976–17985. [Google Scholar] [CrossRef] [PubMed]
- Coucouvanis, D. Useful Reagents and Ligands. In Inorganic Syntheses; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 75–121. [Google Scholar]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Tong, L. 6,6′-Di-(8″-quinoline)-2,2′-bipyridine Cobalt(II) Complex. Molbank 2023, 2023, M1615. https://doi.org/10.3390/M1615
Li Y, Tong L. 6,6′-Di-(8″-quinoline)-2,2′-bipyridine Cobalt(II) Complex. Molbank. 2023; 2023(2):M1615. https://doi.org/10.3390/M1615
Chicago/Turabian StyleLi, Yuwei, and Lianpeng Tong. 2023. "6,6′-Di-(8″-quinoline)-2,2′-bipyridine Cobalt(II) Complex" Molbank 2023, no. 2: M1615. https://doi.org/10.3390/M1615
APA StyleLi, Y., & Tong, L. (2023). 6,6′-Di-(8″-quinoline)-2,2′-bipyridine Cobalt(II) Complex. Molbank, 2023(2), M1615. https://doi.org/10.3390/M1615





