Abstract
The novel heteroleptic copper (I) complex [6-(thiophen-2-yl)-2,2′-bipyridine]bis(triphenylphosphine) copper(I) tetrafluoroborate (1), formulated as [CuL(PPh3)2]BF4, was synthesized in two steps, utilizing the diimine type ligand L = 6-(thiophen-2-yl)-2,2′-bipyridine and triphenylphosphine (PPh3). The compound was characterized both in the solid state and in solution by employing single crystal X-ray diffraction, IR, UV, and NMR spectroscopies. The complex is an orange emitter that demonstrates a photoluminescence quantum yield of 2.6% in the solid state.
1. Introduction
Copper(I) heteroleptic complexes bearing phosphines and diimine-type ligands are of great interest because of their attractive photophysical properties. These compounds could be used as phosphorescent active materials in organic, light-emitting devices (OLEDs) and light-emitting electrochemical cells (LECs) [1,2,3,4] as promising alternatives to their already-known heavy metal analogues.
Chelating N^N ligands based on 2,2′-bipyridine (bpy) and 1,10−phenanthroline cores are the most widely used because of their rigidity and strong binding to Cu(I). On the other hand, the phosphine ligand, which acts as a good σ-donor and π-acceptor, stabilizes the metal oxidation state. The optimization of the photophysical properties of Cu(NN)(P) complexes requires a careful choice of both the N^N (sterically demanding, rigid, and highly conjugated) and P (preferably bidentate, with a large bite angle) type ligands [5,6,7,8,9].
I. Andres-Tome et al. synthesized a series of [Cu(N^N)(PPh3)2]+-type complexes (in which N^N stands for the 2,2′-bipyridine and methylated derivatives). Cu(I) is tetra-coordinated, exhibiting moderate distortion from the ideal tetrahedral geometry. The compounds were emissive in the solid state (polymer matrix) at room temperature, with low to moderate quantum yields [10].
B. Bozic-Weber and co-workers have already studied the non-emissive homoleptic complex [CuL2][PF6] that carries the ligand used in our work. Notably, one of the Cu-N bonds is longer (2.20 Å) than the remaining ones (~2.00 Å). This elongation is associated with a deformation of the corresponding bpy ligand away from planarity. The electronic absorption spectrum exhibits high energy bands arising from ligand-centered π* ← π transitions. The broad spectral features, observed at approximately 400–435 and 513–550 nm, respectively, were assigned to MLCT transitions [11].
A heteroleptic complex of Cu(I) with this ligand and a phosphine has not yet been reported. Keeping in mind the absorption properties of the homoleptic complex mentioned above and the expected effect of the 2-2′-bpy 6-position substitution with a thiophenyl moiety (i.e., a conjugation extension and redshift of the emission maximum), we decided to synthesize and study the title compound.
2. Results and Discussion
2.1. Characterization with NMR Spectroscopy
The 1H-NMR spectrum of the complex 1 (Figure 1) was recorded in acetone-d6. One set of sharp signals was observed, which was indicative of the complex integrity in the solution. The 1H chemical shifts of both the ligand and the complex are cited in Table 1. The proton resonance assigned to H(6′) was most affected by complexation and was shifted by 1 ppm upfield compared to that of the free ligand. Notably, upfield shifts were also observed for the thiophene protons, implying the presence of intramolecular stacking possibly involving the thiophene moiety and the phenyl rings of the phosphine ligands [12,13,14].
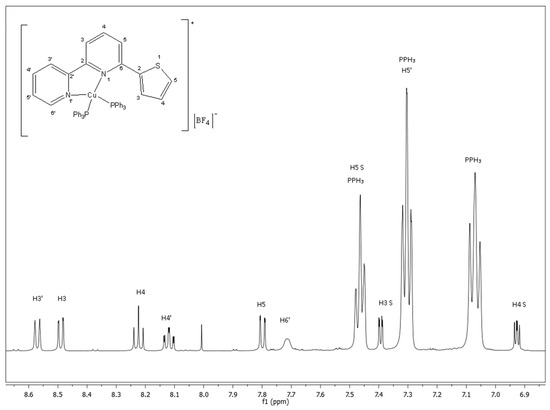
Figure 1.
1HNMR spectrum of [CuL(PPh3)2][BF4] (1) in acetone-d6 (500 MHz, 298K).

Table 1.
1H NMR data (δ, ppm) for L and the complex 1 ([CuL(PPh3)2][BF4]).
2.2. Absorption Spectrum
The diffuse reflectance spectrum (DRS) of the [CuL(PPh3)2]BF4 (1) is shown in Figure 2. The broad spectral features present from 250–480 nm are expected for complexes of this type. More specifically, the ligand-centered (LC) π → π* and n → π* electronic transitions appeared in the range of 230–350 nm, while lower energy bands (>350 nm) are ascribed to MLCT transitions [15,16,17].
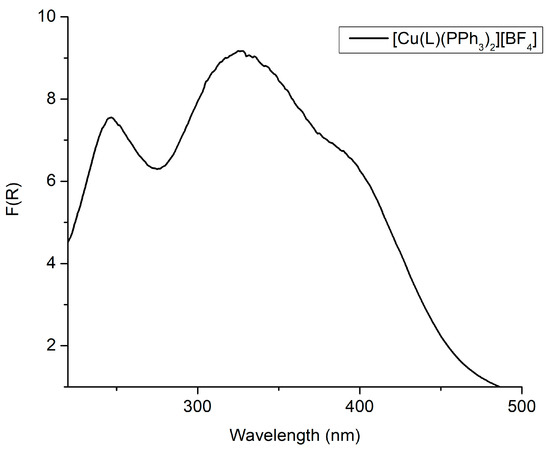
Figure 2.
The Kubelka–Munk spectrum of the solid [CuL(PPh3)2]BF4.
2.3. IR Spectroscopy
The ATR-IR spectrum of the Cu(I) complex 1, presented in Figure 3, reveals the presence of both ligands and the counter ion. The most characteristic bands were located at 1594, 1460, and 1053 cm−1 and are assigned to C=N, =C-P, and B-F stretching vibrations, respectively [16].
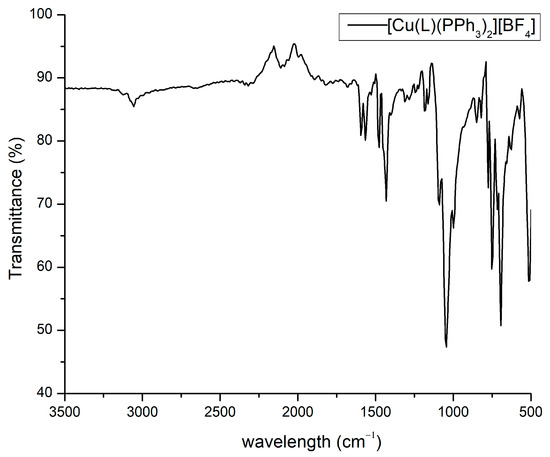
Figure 3.
The ATR-IR spectrum of the complex 1.
2.4. Emission Spectrum–Quantum Yield Calculation
Complex 1 exhibited no luminescence in solution. Nevertheless, a broad emission band located at 600 nm (orange), appeared upon excitation at λ = 350 nm at room temperature (Figure 4). The large Stoke’s shift (~250 nm) implies a severe energy loss in the MLCT excited state, most probably due to structural relaxation [15,16,17]. This also accounts for the relatively low calculated absolute photoluminescence quantum yield of Φ = 2.6%.
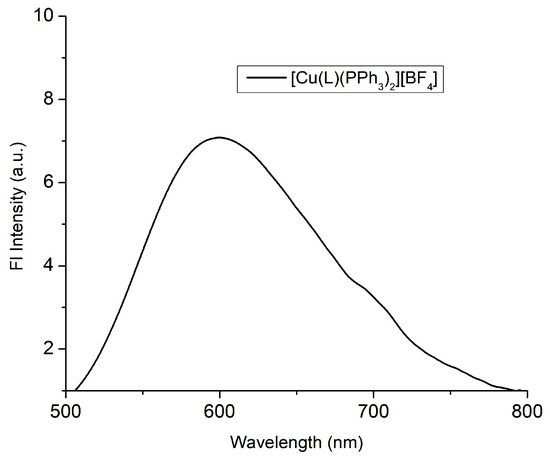
Figure 4.
The emission spectrum of the solid [CuL(PPh3)2]BF4 (λexc = 350 nm).
2.5. Description of the Structure
The compound is formulated as [CuL(PPh3)2]BF4 (1), where L is 6-(thiophen-2-yl)-2,2′-bipyridine, and is crystallized in the monoclinic space group P21/c. Its asymmetric unit consists of a cation [CuL(PPh3)2]+ and the counter anion BF4−. Selected bond distances (Å) and angles (°) for the coordination sphere of Cu(I) in the cation are presented in Table 2, and the structure of the cation is depicted in Figure 5.

Table 2.
Selected structural characteristics of compound 1.
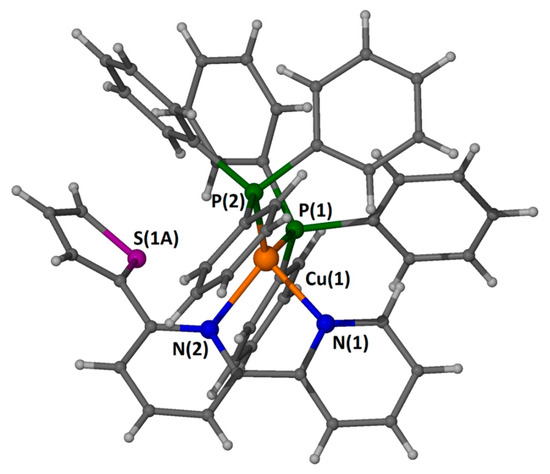
Figure 5.
A “ball and stick” presentation of the cation in compound 1 with a partial labeling scheme. Only one part of the thiophenyl moiety is shown for clarity.
Compound 1 joins a small family of structurally characterized compounds formulated as [Cu(N-N chelate)(unidentate phosphine)2](counter anion), and it is the first example containing the ligand 6-(thiophen-2-yl)-2,2′-bipyridine [18]. In the cation, Cu(1) sits in the center of a rather distorted tetrahedron built by two nitrogen atoms [N(1) and N(2)] that belong to a chelated 6-(thiophen-2-yl)-2,2′-bipyridine ligand and two phosphorus atoms [P(1) and P(2)] that belong to two coordinated PPh3 molecules. The distortions of the tetrahedron can be easily seen from the wide P(1)-Cu(1)-P(2) angle [120.46(2)°] due to the large steric hindrance of the phenyl groups, and from the acute N(1)-Cu(1)-N(2) angle [78.93(8)°] due to the small bite of the chelation. The Cu-P and Cu-N bond distances agree with literature values [19,20,21]. The heterocyclic ligand utilizes only the pyridyl moieties for coordination. The two rings are not coplanar, forming a dihedral angle of 21.32°. The thiophenyl group is disordered, probably due to the lack of strong interactions with the lattice constituents. The BF4− is held in the crystal with non-conventional C-H⋯F H-bonds. A packing diagram is shown in Figure 6.
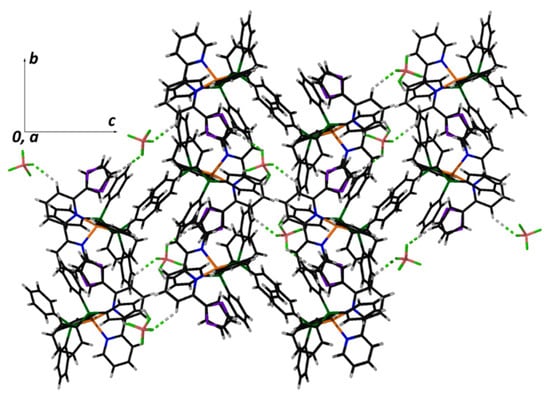
Figure 6.
A packing diagram of 1 down to the a axis of the unit cell. Both positions of the disordered thiophenyl group and the non-conventional H-bonds are shown.
3. Materials and Methods
3.1. Materials
All solvents were of analytical grade and used without further purification. [Cu(CH3CN)4]BF4, PPh3, and NH4OAc were purchased from Aldrich, while 2-acetylthiophene, 2-acetylpyridine, and Me2NH.HCl were purchased from Alfa Aesar. I2 and paraformaldehyde were purchased from Riedel de Haen. The ligand L = 6-(thiophen-2-yl)-2,2′-bipyridine was prepared according to a reported method [12].
3.2. Methods
The high-resolution electrospray ionization mass spectrum (HR-ESI-MS) of the compound (Figure S2) was obtained on a Thermo Scientific, LTQ Orbitrap XL™ system. A C, H, and N elemental analysis was performed on a Perkin-Elmer 2400 Series II analyzer. 1H and 1H-1H-COSY NMR spectra (Figure S1) were recorded on Bruker Avance spectrometer operating at proton frequencies of 400.13 and 500.13 MHz and processed using Topspin 4.07 (Bruker Biospin GmbH, Ettlingen, Germany). The IR spectrum of the complex was acquired on an Agilent Cary 630 ATR-IR spectrometer. The DRS-absorbance spectrum of the complex was recorded on an Agilent Cary 60 UV–vis spectrophotometer with a xenon source lamp equipped equipped with an external reflectance probe (Barrelino™, Harrick Scientific Products, Inc., New York, NY, USA). The emission study was carried out using a Jasco FP-8300 fluorometer equipped with a xenon lamp source and an integrated sphere for solid samples. The photoluminescence absolute quantum yield of the complex in the solid state was calculated using the equation Q = S2/S0 − S1. S2 denotes the integrated emission intensity of the sample, while S0 and S1 represent the integrated excitation intensities of the standard and the sample, respectively.
3.3. Crystal Structure Determination
A suitable, prismatic yellow crystal of compound 1 with approximate dimensions of 0.40 × 0.40 × 0.50 mm3 was glued to a thin glass fiber with a cyanoacrylate (super glue) adhesive and placed on the goniometer head. Diffraction data were collected on a Bruker D8 Quest Eco diffractometer equipped with a Photon II detector and a TRIUMPH (curved graphite) monochromator utilizing Mo Ka radiation (λ = 0.71073 Å), using the APEX 3 software package [22]. A total of 555 frames were collected with φ and ω scans. The collected frames were integrated with the Bruker SAINT software using a wide-frame algorithm. The integration of the data using a monoclinic unit cell yielded a total of 140,512 reflections to a maximum θ angle of 25.00° (0.84 Å resolution). Of these, 7632 were independent (average redundancy = 18.411, completeness = 99.8%, Rint = 4.60%, and Rsig = 1.52%), and 6559 (85.94%) were greater than 2σ(F2). The final cell constants of a = 13.0988(5), b = 16.1147(6), c = 20.7612(7) Å, β = 98.2330(10)°, and V = 4337.2(3) Å3 are based upon the refinement of the XYZ centroids of 9727 reflections above 20 σ(I) with 5.055° < 2θ < 59.67°. The data were corrected for absorption effects using the multi-scan method (SADABS) [23]. The ratio of the minimum to the maximum apparent transmission was 0.887. The P21/c space group was assigned, and the structure was solved using the Bruker SHELXT Software Package. It was then refined by full-matrix least-squares techniques on F2 (SHELXL 2018/3) [24] via the ShelXle interface [25] A few reflections below theta (min.) were missing, probably because they were affected by the beam stop. Further experimental crystallographic details for 1: Data/restraints/parameters, 7632/140/587; (Δ/σ)max = 0.001; (Δρ)max/(Δρ)min = 0.726/−0.379 eÅ−3; goodness of fit, 1.065; R indices [I > 2σ(I)], Robs = 0.0336, wRobs = 0.0899; R indices [all data], Rall = 0.0430, and wRall = 0.0951. The non-H atoms were treated anisotropically, while the organic H atoms were placed in calculated, ideal positions and refined as riding on their respective carbon atoms. PLATON [26] was used for geometric calculations, and X-Seed [27] was used for the molecular graphics.
The thiophenyl group was treated as disordered in two positions, which were related with an approximately 180° rotation about the pyridyl-thiophenyl ring bond, and partial occupancies of 76 and 24%.
Full details on the structure can be found in the CIF file deposited with the CCDC. CCDC 2241976 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif (accessed on 15 February 2023).
3.4. Synthesis
Synthesis of the Complex [Cu(L)(PPh3)2][BF4] (1)
In a 25 mL round bottom flask, 5 mL of dichloromethane (Ar-degassed), 31.4 mg of [Cu(CH3CN)4]+[BF4]− (0.1 mmol), and 52.5 mg of PPh3 (0.2 mmol) were added. The solution was stirred for an hour. Then, 23.8 mg of L (0.1 mmol) was added, and the orange solution was left to stir overnight. The solvent was removed under reduced pressure up to 1ml, followed by the addition of diethyl ether. A yellow-color solid immediately precipitated and was collected and dried under vacuum. Yield: 54.7% (m = 50 mg). C50H40N2BSF4P2Cu: calc.% C, 65.76; H, 4.42; N, 3.07. Found C, 65.70; H, 4.44; N, 3.11. 1H NMR (500 MHz, acetone-d6) δ (ppm) L: 8.57 (d, J = 8.2 Hz, 1H); 8.49 (dd, J = 8.0 Hz, 0.7 Hz, 1H); 8.23 (t, J = 7.9 Hz, 1H); 8.12 (td, J = 7.9 Hz, 1.6 Hz, 1H); 7.80 (dd, J = 7.9 Hz, 0.8 Hz, 1H); 7.71 (br, 1H); 7.46 (t, J = 7.1 Hz, 1H); 7.40 (dd, J = 5.0Hz, 1.1 Hz, 1H); 7.31 (t, J = 7.9 Hz, 1H); 6.93 (dd, J = 5.0 Hz, 3.6 Hz, 1H). PPh3: 7.46, 7.30, 7.07 (30H). AΤR-IR (cm−1) 3053w (=C-H aromatic), 1594m (C=N bipyridyl), 1563m (C=C aromatic), 1460m (=C-P, PPh3) 1053s (B-F BF4). HR ESI MS: cal. m/z = 300.9855, found m/z = 300.9839 for C14H10CuN2S [Cu(L)]+; cal. m/z = 563.0767, found m/z = 563.0736 for C32H25CuN2PS [Cu(L)(PPh3)]+.
4. Conclusions
In summary, we synthesized and structurally characterized a novel heteroleptic Cu(I) complex bearing a 2,2′-bipyridine type ligand and triphenylphosphine. The compound is emissive in the solid state at room temperature, showing a photoluminescence quantum yield of 2.6%.
Supplementary Materials
The following supporting information can be downloaded online, Figure S1: 1H-1H-COSY NMR spectrum of the complex; Figure S2: HR ESI-MS spectrum of the complex in acetone (top) and theoretical spectrum for the fragments [CuL2]+ (middle) and [CuLP]+ (bottom); File S1: crystal structure of the compound (*.mol file). File GM5_22_0m.cif (*.cif file)
Author Contributions
Conceptualization: G.M.; supervision: G.M.; formal analysis: P.K., D.G., and J.C.P.; investigation: P.K. and D.G.; X-ray crystallography: J.C.P.; writing—original draft: D.G. and J.C.P.; writing—review & editing: G.M and J.C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
CCDC 2241976 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data in this study can be found in Supplementary Materials.
Acknowledgments
The authors would like to thank The Network of Research Supporting Laboratories at the University of Ioannina for providing access to use MS, NMR and X-ray diffraction facilities.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Min, J.; Zhang, Q.; Sun, W.; Cheng, Y.; Wang, L. Neutral copper(i) phosphorescent complexes from their ionic counterparts with 2-(2′-quinolyl)benzimidazole and phosphine mixed ligands. Dalton Trans. 2010, 40, 686–693. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, J.; Cheng, Y.; Wang, L.; Xie, Z.; Jing, X.; Wang, F. Novel Heteroleptic CuI Complexes with Tunable Emission Color for Efficient Phosphorescent Light-Emitting Diodes. Adv. Funct. Mater. 2007, 17, 2983–2990. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Q.; Cheng, Y.; Wang, L.; Ma, D.; Jing, X.; Wang, F. Highly Efficient Electroluminescence from Green-Light-Emitting Electrochemical Cells Based on CuI Complexes. Adv. Funct. Mater. 2006, 16, 1203–1208. [Google Scholar] [CrossRef]
- Armaroli, N.; Accorsi, G.; Holler, M.; Moudam, O.; Nierengarten, J.-F.; Zhou, Z.; Wegh, R.T.; Welter, R. Highly Luminescent CuI Complexes for Light-Emitting Electrochemical Cells. Adv. Mater. 2006, 18, 1313–1316. [Google Scholar] [CrossRef]
- Safin, D.A.; Mitoraj, M.P.; Robeyns, K.; Filinchuk, Y.; Velde, C.M.L.V. Luminescent mononuclear mixed ligand complexes of copper(i) with 5-phenyl-2,2′-bipyridine and triphenylphosphine. Dalton Trans. 2015, 44, 16824–16832. [Google Scholar] [CrossRef]
- Li, G.F.; Zhang, X.Y.; Li, R.F.; Liu, X.F. Synthesis and properties of two new Cu(I) complexes based on 5,6-substituted imidazole-2,9-dimethyl-1,10-phenanthroline and triphenylphosphine. Russ. J. Gen. Chem. 2016, 86, 387–390. [Google Scholar] [CrossRef]
- Steen, R.O.; Nurkkala, L.J.; Angus-Dunne, S.J.; Schmitt, C.X.; Constable, E.C.; Riley, M.J.; Bernhardt, P.V.; Dunne, S.J. The Role of Isomeric Effects on the Luminescence Lifetimes and Electrochemistry of Oligothienyl-Bridged Dinuclear Tris(2,2′-bipyridine)ruthenium(II) Complexes. Eur. J. Inorg. Chem. 2008, 2008, 1784–1794. [Google Scholar] [CrossRef]
- Leoni, E.; Mohanraj, J.; Holler, M.; Mohankumar, M.; Nierengarten, I.; Monti, F.; Sournia-Saquet, A.; Delavaux-Nicot, B.; Nierengarten, J.-F.; Armaroli, N. Heteroleptic Copper(I) Complexes Prepared from Phenanthroline and Bis-Phosphine Ligands: Rationalization of the Photophysical and Electrochemical Properties. Inorg. Chem. 2018, 57, 15537–15549. [Google Scholar] [CrossRef]
- Beaudelot, J.; Oger, S.; Peruško, S.; Phan, T.-A.; Teunens, T.; Moucheron, C.; Evano, G. Photoactive Copper Complexes: Properties and Applications. Chem. Rev. 2022, 122, 16365–16609. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Tomé, I.; Fyson, J.; Dias, F.B.; Monkman, A.P.; Iacobellis, G.; Coppo, P. Copper(i) complexes with bipyridyl and phosphine ligands: A systematic study. Dalton Trans. 2012, 41, 8669–8674. [Google Scholar] [CrossRef]
- Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E.; Kopecky, P.; Neuburger, M.; Zampese, J.A. The intramolecular aryl embrace: From light emission to light absorption. Dalton Trans. 2011, 40, 12584–12594. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-K.; Zhao, L.-X.; Basnet, A.; Thapa, P.; Karki, R.; Na, Y.; Jahng, Y.; Jeong, T.C.; Jeong, B.-S.; Lee, C.-S.; et al. Synthesis of 2,6-diaryl-substituted pyridines and their antitumor activities. Eur. J. Med. Chem. 2008, 43, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Henney, R.P.G.; Leese, T.A.; Tocher, D.A. Cyclometallation reactions of 6-phenyl-2,2′-bipyridine; a potential C,N,N-donor analogue of 2,2′: 6′,2″-terpyridine. Crystal and molecular structure of dichlorobis(6-phenyl-2,2′-bipyridine)ruthenium(II). J. Chem. Soc. Dalton Trans. 1990, 2, 443–449. [Google Scholar] [CrossRef]
- Constable, E.C.; Henney, R.P.G.; Tocher, D.A. Co-ordination chemistry of 2-phenyl-6-(2-thienyl)pyridine and 2,6-bis(2-thienyl)pyridine; new ambidentate ligands. J. Chem. Soc. Dalton Trans. 1992, 16, 2467–2474. [Google Scholar] [CrossRef]
- Zhang, L.; Zuo, Q. A series of blue-green-yellow-red emitting Cu(I) complexes: Molecular structure and photophysical performance. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117280. [Google Scholar] [CrossRef]
- Shi, L.; Li, B.; Lu, S.; Zhu, D.; Li, W. Synthesis, characterization and oxygen-sensing properties of a novel luminescent Cu(I) complex. Appl. Organomet. Chem. 2009, 23, 379–384. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Liu, W.; Qin, W. Study on the synthesis, characterization, photophysical performance and oxygen-sensing behavior of a luminescent Cu(I) complex with large conjugation plane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 104, 56–63. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Lin, Y.-R.; Huang, J.-S.; Zhong, M.-H. [6-(4-Bromophenyl)-2,2′-bipyridine-κ2N,N′]bis(triphenylphosphine-κP)copper(I) tetrafluoridoborate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, m1187. [Google Scholar] [CrossRef]
- Tikkanen, W.R.; Kueger, C.; Bomben, K.D.; Jolly, W.L.; Kaska, W.; Ford, P.C. Synthesis, characterization, and x-ray molecular structures of mono- and dinuclear copper complexes with 2,7-bis(2-pyridyl)-1,8-naphthyridine. Inorg. Chem. 1984, 23, 3633. [Google Scholar] [CrossRef]
- Sequeira, D.; Baptista, P.V.; Valente, R.; Piedade, M.F.M.; Garcia, M.H.; Morais, T.S.; Fernandes, A.R. Cu(I) complexes as new antiproliferative agents against sensitive and doxorubicin-resistant colorectal cancer cells: Synthesis, characterization, and mechanisms of action. Dalton Trans. 2021, 50, 1845. [Google Scholar] [CrossRef]
- Bruker, APEX 3. In SAINT, SHELXT; Bruker AXS Inc.: Fitchburg, WI, USA, 2016.
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.J. X-Seed—A Software Tool for Supramolecular Crystallography. J. Supramol. Chem. 2001, 1, 189–191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).