2,9-Dimethyl-4H-oxazolo[5’,4’:4,5]pyrano[3,2-f]quinolin-4-one
Abstract
1. Introduction
2. Results and Discussion
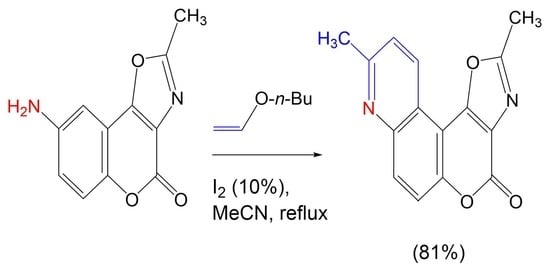
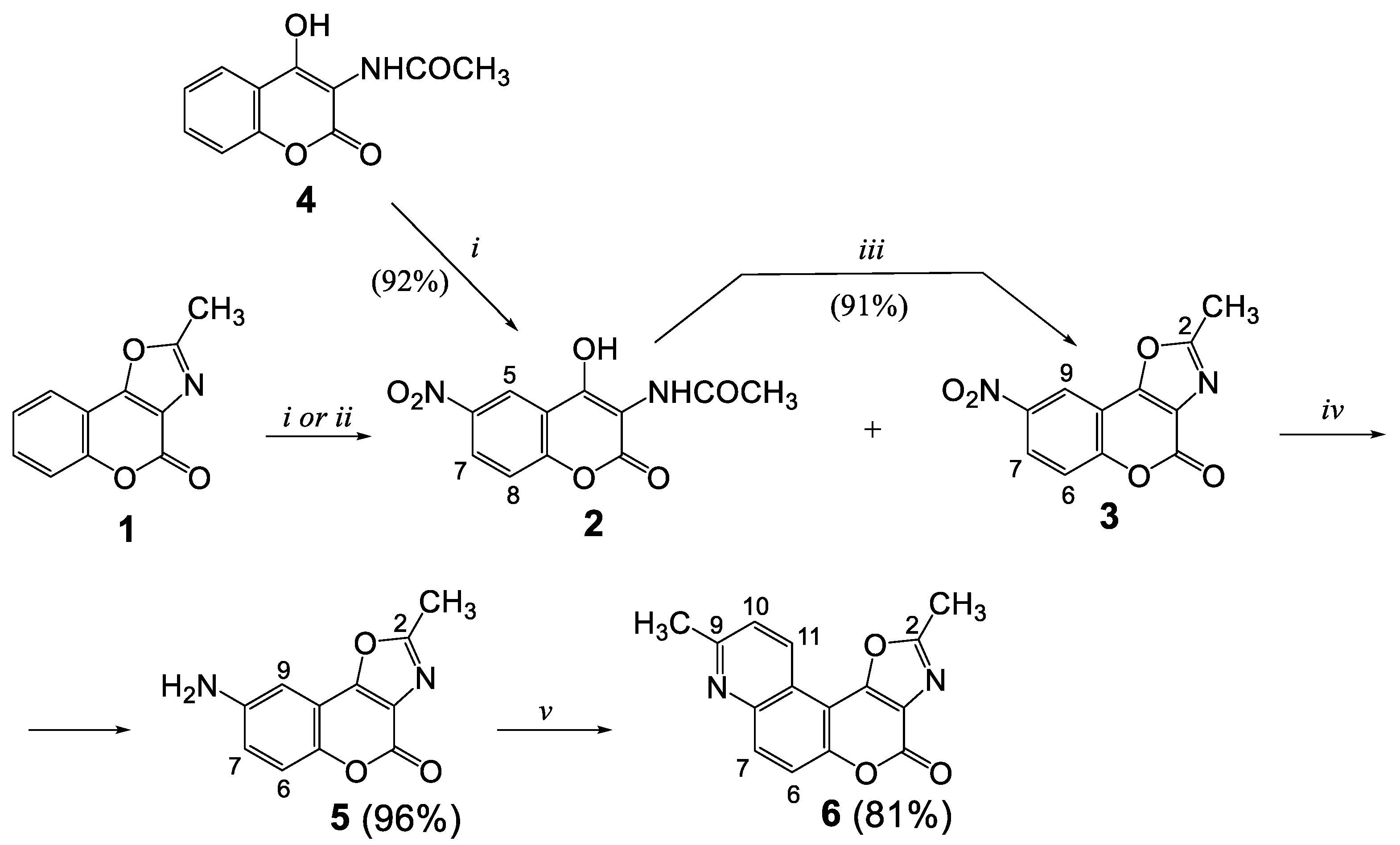
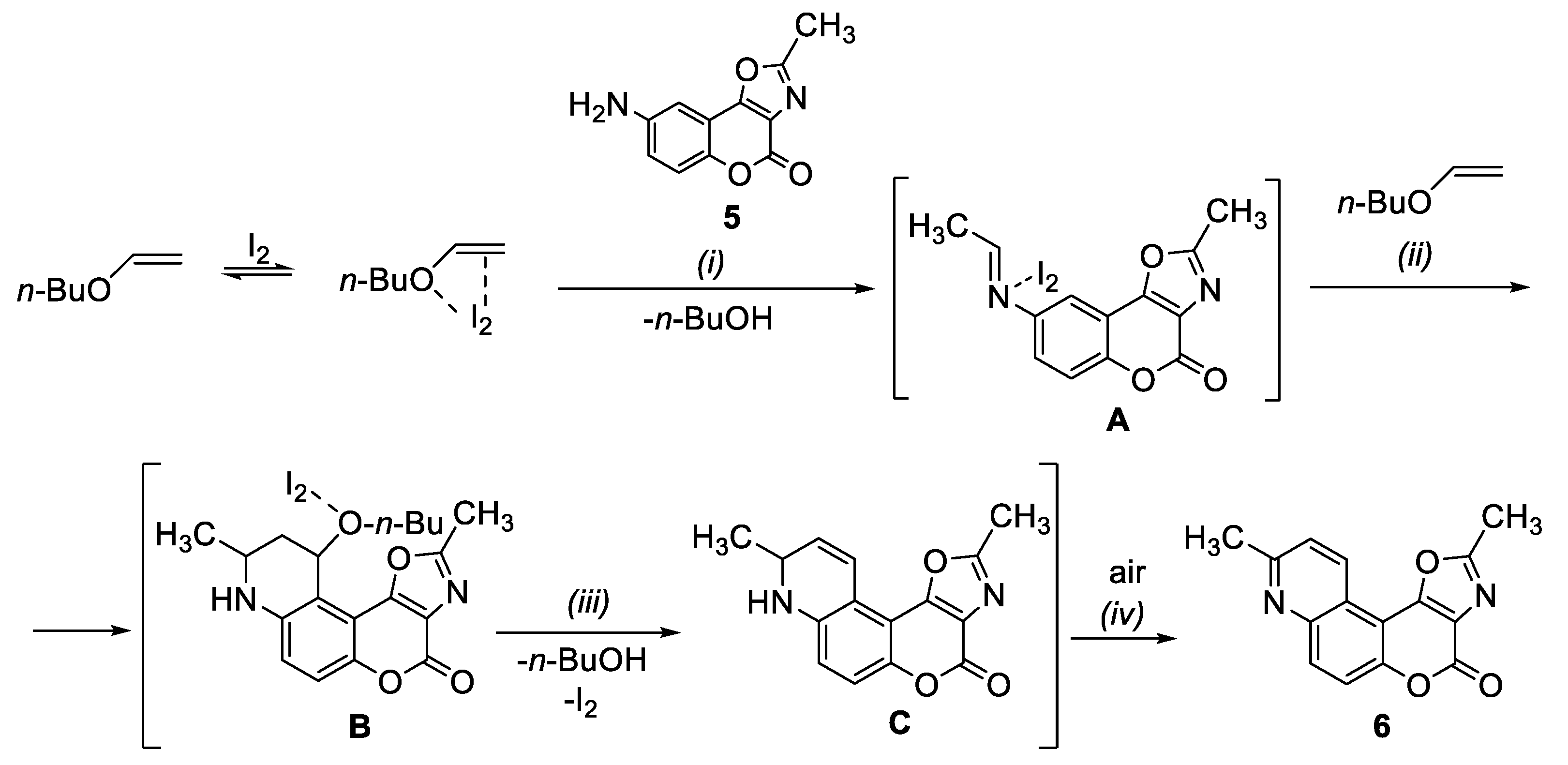
2.1. Synthesis
2.2. Biology
3. Materials and Methods
3.1. Materials
3.2. Nitration of 2-Methyl-4H-chromeno[3,4-d]oxazol-4-one (1) Synthesis of N-(4-hydroxy-6-nitro-2-oxo-2H-chromen-3-yl)acetamide (2) and 2-methyl-8-nitro-4H-chromeno[3,4-d]oxazol-4-one (3)
3.3. Nitration of N-(4-Hydroxy-2-oxo-2H-chromen-3-yl)acetamide (4) Synthesis of N-(4-hydroxy-6-nitro-2-oxo-2H-chromen-3-yl)acetamide (2)
3.4. Condensation of N-(4-Hydroxy-6-nitro-2-oxo-2H-chromen-3-yl)acetamide (2) Synthesis of 2-Methyl-8-nitro-4H-chromeno[3,4-d]oxazol-4-one (3)
3.5. Synthesis of 8-Amino-2-methyl-4H-chromeno[3,4-d]oxazol-4-one (5)
3.6. Synthesis of 2,9-Dimethyl-4H-oxazolo[5’,4’:4,5]pyrano[3,2-f]quinolin-4-one (6)
3.7. Biological Experiments: In Vitro Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, D.L.; Suzuki, M.; Xie, L.; Morris-Natsche, S.L.; Lee, K.H. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med. Res. Rev. 2003, 23, 322–345. [Google Scholar] [CrossRef] [PubMed]
- Fylaktakidou, K.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolaides, D. Natural and Synthetic Coumarin Derivatives with Anti-Inflammatory/Antioxidant Activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Lacy, A.; O’Kennedy, R. Studies on Coumarins and Coumarin-Related Compounds to Determine their Therapeutic Role in the Treatment of Cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M.; González, M.C.; Córdova-Guerrero, I.; García, A.G.T.; Osegueda-Robles, S. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, Y.; Li, L. Coumarins as potential antidiabetic agents. J. Pharm. Pharmacol. 2017, 69, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Salehian, F.; Nadri, H.; Jalili-Baleh, L.; Youseftabar-Miri, L.; Bukhari, S.N.A.; Foroumadi, A.; Küçükkilinç, T.T.; Sharifzadeh, M.; Khoobi, M. A review: Biologically active 3,4-heterocycle-fused coumarins. Eur. J. Med. Chem. 2020, 212, 113034. [Google Scholar] [CrossRef]
- Kontogiorgis, C.; Hadjipavlou-Litina, D. Biological Evaluation of Several Coumarin Derivatives Designed as Possible Anti-inflammatory/Antioxidant Agents. J. Enzym. Inhib. Med. Chem. 2003, 18, 63–69. [Google Scholar] [CrossRef]
- Prasanna, B.; Sandeep, A.; Revathi, T. Green approach to synthesis of novel substituted 8H-pyrano[2,3-e]benzoxazole-8-ones. World J. Pharm. Pharm. Sci. 2014, 3, 404–411. [Google Scholar]
- Soares, A.M.S.; Hungerford, G.; Gonçalves, M.S.T.; Costa, S.P.G. Light triggering of 5-aminolevulinic acid from fused coumarin ester cages. New J. Chem. 2017, 41, 2997–3005. [Google Scholar] [CrossRef]
- Balalas, T.D.; Stratidis, G.; Papatheodorou, D.; Vlachou, E.-E.; Gabriel, C.; Hadjipavlou-Litina, D.J.; Litinas, K.E. One-pot Synthesis of 2-Substituted 4H-Chromeno[3,4-d]oxazol-4-ones from 4-Hydroxy-3-nitrocoumarin and Acids in the Presence of Triphenylphosphine and Phosphorus Pentoxide under Microwave Irradiation. Synopen 2018, 2, 105–113. [Google Scholar] [CrossRef]
- Douka, M.D.; Litinas, K.E. An Overview on the Synthesis of Fused Pyridocoumarins with Biological Interest. Molecules 2022, 27, 7256. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Kulkarni, M.V.; Gopal, M.; Shahabuddin, M.; Sun, C.-M. Synthesis and biological evaluation of novel angularly fused polycyclic coumarins. Bioorganic Med. Chem. Lett. 2005, 15, 3584–3587. [Google Scholar] [CrossRef] [PubMed]
- Markey, M.D.; Fu, Y.; Kelly, T.R. Synthesis of Santiagonamine. Org. Lett. 2007, 9, 3255–3257. [Google Scholar] [CrossRef] [PubMed]
- El-Saghier, A.M.M.; Naili, M.B.; Rammash, B.K.; Saleh, N.A.; Kreddan, K.M. Synthesis and antibacterial activity of some new fused chromenes. Arkivoc 2007, 16, 83–91. [Google Scholar] [CrossRef]
- Levrier, C.; Balastrier, M.; Beattie, K.; Carroll, A.; Martin, F.; Choomuenwai, V.; Davis, R.A. Pyridocoumarin, aristolactam and aporphine alkaloids from the Australian rainforest plant Goniothalamus australis. Phytochemistry 2013, 86, 121–126. [Google Scholar] [CrossRef]
- Symeonidis, T.S.; Hadjipavlou-Litina, D.J.; Litinas, K.E. Synthesis Through Three-Component Reactions Catalyzed by FeCl3 of Fused Pyridocoumarins as Inhibitors of Lipid Peroxidation. J. Heterocycl. Chem. 2014, 51, 642–647. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Shukla, S.; Nandy, S.; Sahoo, H. Synthesis of novel coumarin derivatives and its biological evaluations. Eur. J. Exp. Biol. 2012, 2, 899–908. [Google Scholar]
- Colotta, V.; Catarzi, D.; Varano, F.; Cecchi, L.; Filacchioni, G.; Martini, C.; Giusti, L.; Lucacchini, A. Tricyclic heteroaromatic systems. Synthesis and benzodiazepine receptor affinity of 2-substituted-1-benzopyrano[3,4-d]oxazol-4-ones, -thiazol-4-ones, and -imidazol-4-ones. Il Farm. 1998, 53, 375–381. [Google Scholar] [CrossRef]
- Nofal, Z.M.; El-Zahar, M.I.; El-Karim, A. Novel Coumarin Derivatives with Expected Biological Activity. Molecules 2000, 5, 99–113. [Google Scholar] [CrossRef]
- Dallacker, F.; Kratzer, P.; Lipp, M. Derivate des 2.4-Pyronons und 4-Hydroxy-cumarins. Eur. J. Org. Chem. 1961, 643, 97–109. [Google Scholar] [CrossRef]
- Gammon, D.W.; Hunter, R.; Wilson, S.A. An efficient synthesis of 7-hydroxy-2,6-dimethylchromeno[3,4-d]oxazol-4-one—A protected fragment of novenamine. Tetrahedron 2005, 61, 10683–10688. [Google Scholar] [CrossRef]
- Saikachi, H.; Ichikawa, M. Studies on synthesis of coumarin derivatives. XV. On the preparation of ethyl pyranobenzoxazole-carboxylates. Chem. Pharm. Bull. 1966, 14, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Chantegrel, B.; Nadi, A.I.; Gelin, S. Synthesis of [1]benzopyrano[3,4-d]isoxazol-4-ones from 2-substituted chromone-3-carboxylic esters. A reinvestigation of the reaction of 3-acyl-4-hydroxycoumarins with hydroxylamine. Synthesis of 4-(2-hydroxybenzoyl)isoxazol-5-ones. J. Org. Chem. 1984, 49, 4419–4424. [Google Scholar] [CrossRef]
- Vlachou, E.-E.; Balalas, T.; Hadjipavlou-Litina, D.; Litinas, K. 4-Amino-2-(p-tolyl)-7H-chromeno[5,6-d]oxazol-7-one. Molbank 2021, 2021, M1237. [Google Scholar] [CrossRef]
- Vlachou, E.-E.N.; Armatas, G.S.; Litinas, K.E. Synthesis of Fused Oxazolocoumarins from o -Hydroxynitrocoumarins and Benzyl Alcohol Under Gold Nanoparticles or FeCl3 Catalysis. J. Heterocycl. Chem. 2017, 54, 2447–2453. [Google Scholar] [CrossRef]
- Liska, K.J.; Fentiman, A.F., Jr.; Foltz, R.L. Use of tris-(dipivalomethanato)europium as a shift reagent in the identification of 3-H-pyrano[3,2-f]quinolin-3-one. Tetrahedron Lett. 1970, 11, 4657–4660. [Google Scholar] [CrossRef]
- Kudale, A.A.; Kendall, J.; Miller, D.O.; Collins, J.L.; Bodwell, G.J. Povarov Reactions Involving 3-Aminocoumarins: Synthesis of 1,2,3,4-Tetrahydropyrido[2,3-c]coumarins and Pyrido[2,3-c]coumarins. J. Org. Chem. 2008, 73, 8437–8447. [Google Scholar] [CrossRef]
- Heber, D.; Berghaus, T. Synthesis of 5H-[1]benzopyrano[4,3-b]pyridin-5-ones containing an azacannabinoidal structure. J. Heterocycl. Chem. 1994, 31, 1353–1359. [Google Scholar] [CrossRef]
- Khan, A.T.; Das, D.K.; Islam, K.; Das, P. A simple and expedient synthesis of functionalized pyrido[2,3-c] coumarin derivatives using molecular iodine catalyzed three-component reaction. Tetrahedron Lett. 2012, 53, 6418–6422. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ponra, S.; Ghosh, D.; Taher, A. Multicomponent Aza-Diels–Alder Synthesis of Phenanthrolines and Pyranoquinolines. Synlett 2011, 2011, 104–110. [Google Scholar] [CrossRef]
- Heber, D.; Ivanov, I.C.; Karagiosov, S.K. The Vilmeier reaction in the synthesis of 3-subtituted 5H-[1]benzopyrano[4,3-b]pyridine-5-ones. An unusual pyridine ring closure. J. Heterocycl. Chem. 1995, 32, 505–509. [Google Scholar] [CrossRef]
- Symeonidis, T.S.; Litinas, K.E. Synthesis of methyl substituted [5,6]- and [7,8]-fused pyridocoumarins via the iodine-catalyzed reaction of aminocoumarins with n-butyl vinyl ether. Tetrahedron Lett. 2013, 54, 6517–6519. [Google Scholar] [CrossRef]
- Ahn, S.; Yoon, J.A.; Han, Y.T. Total Synthesis of the Natural Pyridocoumarins Goniothaline A and B. Synthesis 2018, 51, 552–556. [Google Scholar] [CrossRef]
- Symeonidis, T.S.; Kallitsakis, M.G.; Litinas, K.E. Synthesis of [5,6]-fused pyridocoumarins through aza-Claisen rearrangement of 6-propargylaminocoumarins. Tetrahedron Lett. 2011, 52, 5452–5455. [Google Scholar] [CrossRef]
- Symeonidis, T.S.; Lykakis, I.N.; Litinas, K.E. Synthesis of quinolines and fused pyridocoumarins from N-propargylanilines or propargylaminocoumarins by catalysis with gold nanoparticles supported on TiO2. Tetrahedron 2013, 69, 4612–4616. [Google Scholar] [CrossRef]
- Hao, Z.; Zhou, X.; Ma, Z.; Zhang, C.; Han, Z.; Lin, J.; Lu, G.-L. Dehydrogenative Synthesis of Quinolines and Quinazolines via Ligand-Free Cobalt-Catalyzed Cyclization of 2-Aminoaryl Alcohols with Ketones or Nitriles. J. Org. Chem. 2022, 87, 12596–12607. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.-J.; Zhang, S.-L. Synthesis of Quinolines and 2-Functionalized Quinolines by Difluorocarbene Incorporation. Adv. Synth. Catal. 2022, 364, 2157–2162. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlachou, E.-E.N.; Balalas, T.D.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Douka, M. 2,9-Dimethyl-4H-oxazolo[5’,4’:4,5]pyrano[3,2-f]quinolin-4-one. Molbank 2023, 2023, M1591. https://doi.org/10.3390/M1591
Vlachou E-EN, Balalas TD, Hadjipavlou-Litina DJ, Litinas KE, Douka M. 2,9-Dimethyl-4H-oxazolo[5’,4’:4,5]pyrano[3,2-f]quinolin-4-one. Molbank. 2023; 2023(1):M1591. https://doi.org/10.3390/M1591
Chicago/Turabian StyleVlachou, Evangelia-Eirini N., Thomas D. Balalas, Dimitra J. Hadjipavlou-Litina, Konstantinos E. Litinas, and Matina Douka. 2023. "2,9-Dimethyl-4H-oxazolo[5’,4’:4,5]pyrano[3,2-f]quinolin-4-one" Molbank 2023, no. 1: M1591. https://doi.org/10.3390/M1591
APA StyleVlachou, E.-E. N., Balalas, T. D., Hadjipavlou-Litina, D. J., Litinas, K. E., & Douka, M. (2023). 2,9-Dimethyl-4H-oxazolo[5’,4’:4,5]pyrano[3,2-f]quinolin-4-one. Molbank, 2023(1), M1591. https://doi.org/10.3390/M1591