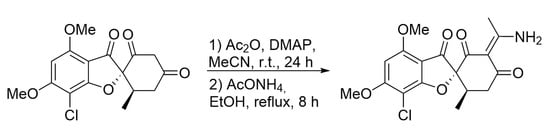
(2R,6′R,E)-3′-(1-Aminoethylidene)-7-chloro-4,6-dimethoxy-6′-methyl-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,3,4′-trione
Abstract
1. Introduction
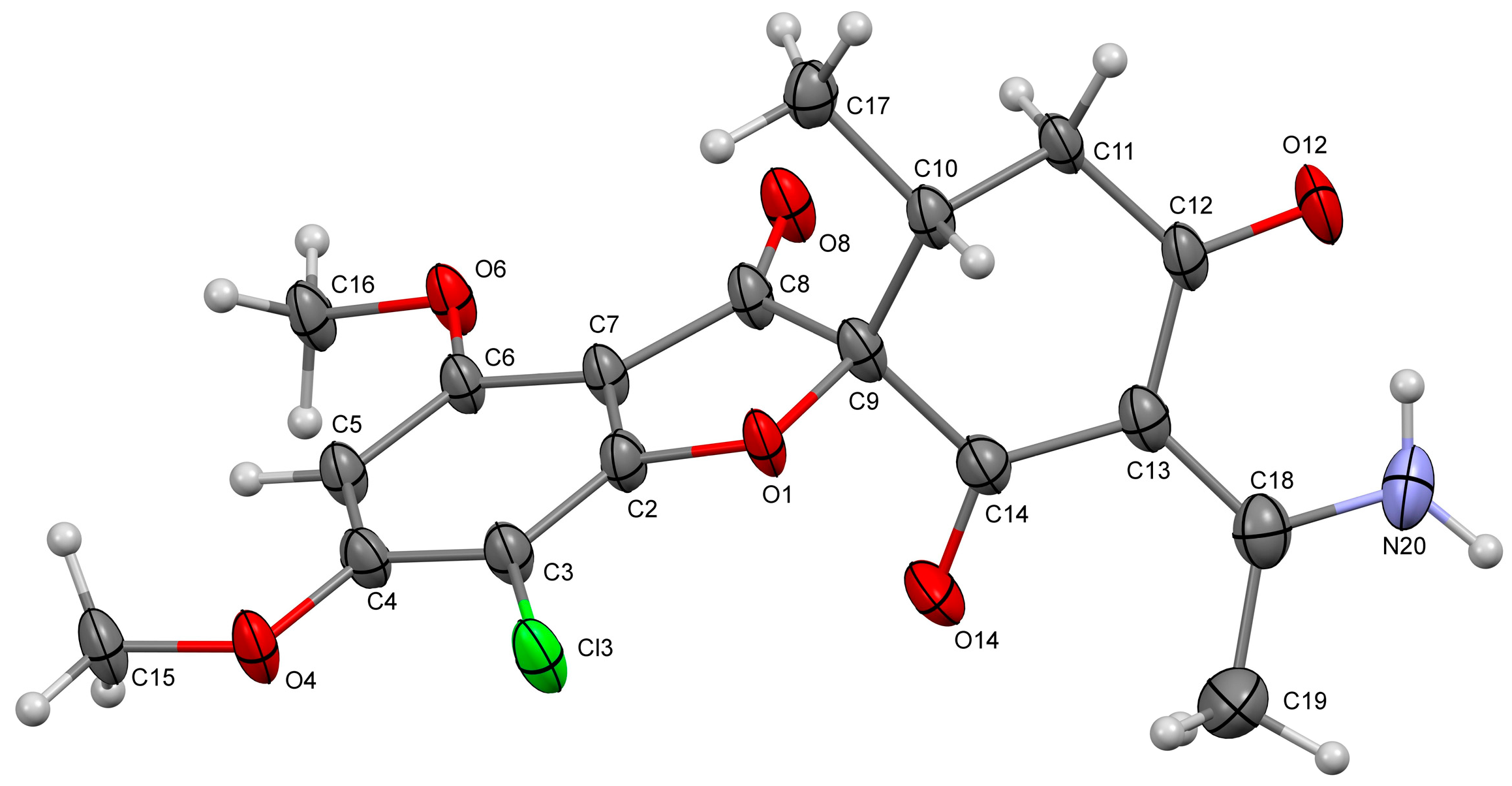
2. Results
3. Materials and Methods
Experimental Procedure for the Synthesis of the (2R,6′R,E)-3′-(1-Aminoethylidene)-7-chloro-4,6-dimethoxy-6′-methyl-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,3,4′-trione 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, C.M.; Roberson, J.S.; Harris, T.M. Biosynthesis of griseofulvin. J. Am. Chem. Soc. 1976, 98, 5380–5386. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.B.; Rønnest, M.H.; Larsen, T.O.; Clausen, M.H. The Chemistry of Griseofulvin. Chem. Rev. 2014, 114, 12088–12107. [Google Scholar] [CrossRef] [PubMed]
- Kartsev, V.; Geronikaki, A.; Petrou, A.; Lichitsky, B.; Kostic, M.; Smiljkovic, M.; Soković, M.; Sirakanyan, S. Griseofulvin Derivatives: Synthesis, Molecular Docking and Biological Evaluation. Curr. Top. Med. Chem. 2019, 19, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Aris, P.; Wei, Y.; Mohamadzadeh, M.; Xia, X. Griseofulvin: An Updated Overview of Old and Current Knowledge. Molecules 2022, 27, 7034. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.-S.; Oritani, T.; Yamashita, K. Synthesis and Biological Activities of Griseofulvin Analogs. Agric. Biol. Chem. 1990, 54, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Brescini, L.; Fioriti, S.; Morroni, G.; Barchiesi, F. Antifungal Combinations in Dermatophytes. J. Fungi 2021, 7, 727. [Google Scholar] [CrossRef] [PubMed]
- Lana, A.J.D.; Pippi, B.; Carvalho, A.R.; Moraes, R.C.; Kaiser, S.; Ortega, G.G.; Fuentefria, A.M.; Silveira, G.P. In Vitro additive effect on griseofulvin and terbinafine combinations against multidrug-resistant dermatophytes. Braz. J. Pharm. Sci. 2018, 54, e17149. [Google Scholar] [CrossRef]
- Shehabeldine, A.; El-Hamshary, H.; Hasanin, M.; El-Faham, A.; Al-Sahly, M. Enhancing the Antifungal Activity of Griseofulvin by Incorporation a Green Biopolymer-Based Nanocomposite. Polymers 2021, 13, 542. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-B.; Gao, Y.-Q.; Nie, X.-D.; Tuong, T.-M.-L.; Li, D.; Gao, J.-M. Antifungal Activity of Griseofulvin Derivatives against Phytopathogenic Fungi in Vitro and in Vivo and Three-Dimensional Quantitative Structure–Activity Relationship Analysis. J. Agric. Food Chem. 2019, 67, 6125–6132. [Google Scholar] [CrossRef]
- El-Nakeeb, M.A.; McLellan, W.L.; Lampen, J.O. Antibiotic Action of Griseofulvin on Dermatophytes. J. Bacteriol. 1965, 89, 557–563. [Google Scholar] [CrossRef]
- Hamdy, A.K.; Sheha, M.M.; Abdel-Hafez, A.A.; Shouman, S.A. Design, Synthesis, and Cytotoxicity Evaluation of Novel Griseofulvin Analogues with Improved Water Solubility. Int. J. Med. Chem. 2017, 2017, 7386125. [Google Scholar] [CrossRef] [PubMed]
- Geronikaki, A.; Kartsev, V.; Petrou, A.; Akrivou, M.G.; Vizirianakis, I.S.; Chatzopoulou, F.M.; Lichitsky, B.; Sirakanyan, S.; Kostic, M.; Smiljkovic, M.; et al. Antibacterial activity of griseofulvin analogues as an example of drug repurposing. Int. J. Antimicrob. Agents 2020, 55, 105884. [Google Scholar] [CrossRef] [PubMed]
- Arkley, V.; Attenburrow, J.; Gregory, G.I.; Walker, T. 241. Griseofulvin Amlogues. Part I. Modification of the aromatic ring. J. Chem. Soc. 1962, 1260–1268. [Google Scholar] [CrossRef]
- Crowe, L.B.; Hughes, P.F.; Alcorta, D.A.; Osada, T.; Smith, A.P.; Totzke, J.; Loiselle, D.R.; Lutz, I.D.; Gargesha, M.; Roy, D.; et al. A Fluorescent Hsp90 Probe Demonstrates the Unique Association between Extracellular Hsp90 and Malignancy in Vivo. ACS Chem. Biol. 2017, 12, 1047–1055. [Google Scholar] [CrossRef]
- Goodwin, D.; Simerska, P.; Chang, C.-H.; Mansfeld, F.M.; Varamini, P.; D’Occhio, M.J.; Toth, I. Active immunisation of mice with GnRH lipopeptide vaccine candidates: Importance of T helper or multi-dimer GnRH epitope. Bioorg. Med. Chem. 2014, 22, 4848–4854. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.F. Indazolyl- and Indolyl-Benzamide Derivatives. WIPO Patent WO2016040809 A1, 17 March 2016. [Google Scholar]
- Chu, D.T.W.; Huckin, S.N. Chemistry of hexamethyldisilazane. Silylation of β-diketones and amination of β-triketones. Can. J. Chem. 1980, 58, 138–142. [Google Scholar] [CrossRef]
- Rønnest, M.H.; Harris, P.; Gotfredsen, C.H.; Larsen, T.O.; Clausen, M.H. Synthesis and single crystal X-ray analysis of two griseofulvin metabolites. Tetrahedron Lett. 2010, 51, 5881–5882. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.41.106a; Rigaku Oxford Diffraction: Warriewood, NSW, Australia, 2021.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lichitsky, B.V.; Komogortsev, A.N.; Melekhina, V.G. (2R,6′R,E)-3′-(1-Aminoethylidene)-7-chloro-4,6-dimethoxy-6′-methyl-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,3,4′-trione. Molbank 2023, 2023, M1590. https://doi.org/10.3390/M1590
Lichitsky BV, Komogortsev AN, Melekhina VG. (2R,6′R,E)-3′-(1-Aminoethylidene)-7-chloro-4,6-dimethoxy-6′-methyl-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,3,4′-trione. Molbank. 2023; 2023(1):M1590. https://doi.org/10.3390/M1590
Chicago/Turabian StyleLichitsky, Boris V., Andrey N. Komogortsev, and Valeriya G. Melekhina. 2023. "(2R,6′R,E)-3′-(1-Aminoethylidene)-7-chloro-4,6-dimethoxy-6′-methyl-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,3,4′-trione" Molbank 2023, no. 1: M1590. https://doi.org/10.3390/M1590
APA StyleLichitsky, B. V., Komogortsev, A. N., & Melekhina, V. G. (2023). (2R,6′R,E)-3′-(1-Aminoethylidene)-7-chloro-4,6-dimethoxy-6′-methyl-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,3,4′-trione. Molbank, 2023(1), M1590. https://doi.org/10.3390/M1590