New Preparation of Ferrocene Carboxylic Acid Benzotriazol-1-yl Ester
Abstract
1. Introduction
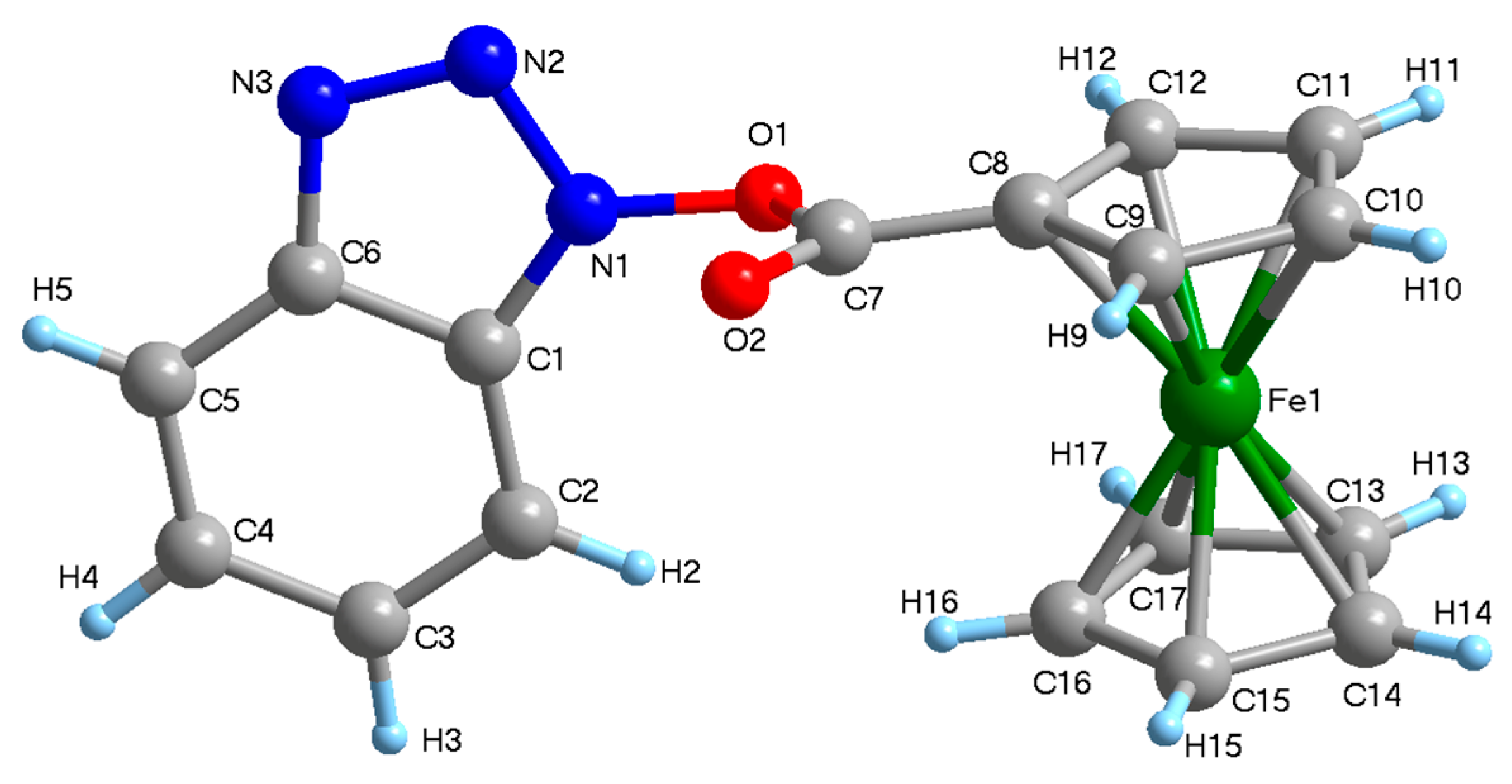
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References and Note
- Miller, S.A.; Tebboth, J.A.; Tremaine, J.F. Dicyclopentadienyliron. J. Chem. Soc. 1952, 632–635. [Google Scholar] [CrossRef]
- Kealy, T.J.; Pauson, P.L. A new type of organo-iron compound. Nature 1951, 168, 1039–1040. [Google Scholar] [CrossRef]
- Pauson, P.L. Ferrocene—how it all began. J. Organomet. Chem. 2001, 637–639, 3–6. [Google Scholar] [CrossRef]
- Fischer, E.O.; Jira, R. How metallocene chemistry and research began in Munich. J. Organomet. Chem. 2001, 637–639, 7–12. [Google Scholar] [CrossRef]
- Rosenblum, M. The early ferrocene days—A personal recollection. J. Organomet. Chem. 2001, 637–639, 13–15. [Google Scholar] [CrossRef]
- Wilkinson, G.; Rosenblum, M.; Whiting, M.C.; Woodward, R.B. The structure of iron bis-cyclopentadienyl. J. Am. Chem. Soc. 1952, 74, 2125–2126. [Google Scholar] [CrossRef]
- Fischer, E.O.; Pfab, W. Cyclopentadien-Metallkomplexe, ein neuer Typ metallorganischer Verbindungen. Z. Naturforschg. 1952, 7, 377–379. Available online: https://zfn.mpdl.mpg.de/data/Reihe_B/7/ZNB-1952-7b-0377.pdf (accessed on 8 December 2022). [CrossRef]
- Eiland, P.F.; Pepinsky, R. X-ray examination of iron biscyclopentadienyl. J. Am. Chem. Soc. 1952, 74, 4971. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Orgel, L.E. Iron bis-cyclopentadienyl: A molecular sandwich. Nature 1953, 171, 121–122. [Google Scholar] [CrossRef]
- Woodward, R.B.; Rosenblum, M.; Whiting, M.C. A new aromatic system. J. Am. Chem. Soc. 1952, 74, 3458–3459. [Google Scholar] [CrossRef]
- Seeman, J.I.; Cantrill, S. Wrong but seminal. Nat. Chem. 2016, 8, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, G. The preparation and some properties of ruthenocene and ruthenicinium salts. J. Am. Chem. Soc. 1952, 74, 6146–6147. [Google Scholar] [CrossRef]
- Heinze, K.; Lang, H. Ferrocene – beauty and function. Organometallics 2013, 32, 5623–5625. [Google Scholar] [CrossRef]
- Gao, D.-W.; Gu, Q.; Zhen, C.; You, S.-L. Synthesis of planar chiral ferrocenes via transition-metal-catalyzed direct C−H bond functionalization. Acc. Chem. Res. 2017, 50, 351–365. [Google Scholar] [CrossRef]
- Martic, S.; Rains, M.K.; Freeman, D.; Kraatz, H.-B. Use of 5′-γ-ferrocenyl adenosine triphosphate (Fc-ATP) bioconjugates having poly(ethylene glycol) spacers in kinase-catalyzed phosphorylations. Bioconj. Chem. 2011, 22, 1663–1672. [Google Scholar] [CrossRef]
- Cao, J.-S.; Zeng, J.; Xiao, J.; Wang, X.-H.; Wang, Y.-W.; Peng, Y. Total synthesis of linoxepin facilitated by Ni-catalyzed tandem reductive cyclization. Chem. Commun. 2022, 58, 7273–7276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Q.; Yan, C.-X.; Xiao, J.; Wang, Y.-W.; Peng, Y. Recent advances on the total synthesis of 2,7′-cyclolignans. Org. Biomol. Chem. 2022, 20, 1623–1636. [Google Scholar] [CrossRef]
- Zhuang, Z.; Luo, Z.; Yao, S.; Wang, Y.; Peng, Y. A concise synthesis of sacidumlignan B. Molecules 2022, 27, 5775. [Google Scholar] [CrossRef]
- Luo, L.; Zhai, X.-Y.; Wang, Y.-W.; Peng, Y.; Gong, H. Divergent total syntheses of C3a-C7′linked diketopiperazine alkaloids (+)-asperazine and (+)-pestalazine A enabled by a Ni-catalyzed reductive coupling of tertiary alkyl chloride. Chem.-Eur. J. 2019, 25, 989–992. [Google Scholar] [CrossRef]
- Xiao, J.; Cong, X.-W.; Yang, G.-Z.; Wang, Y.-W.; Peng, Y. Divergent asymmetric syntheses of podophyllotoxin and related family members via stereoselective reductive Ni-catalysis. Org. Lett. 2018, 9, 3965–3968. [Google Scholar]
- Xiao, J.; Cong, X.-W.; Yang, G.-Z.; Wang, Y.-W.; Peng, Y. Stereoselective synthesis of Podophyllum lignans core by intramolecular reductive nickel-catalysis. Chem. Commun. 2018, 54, 2040–2043. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xiao, J.; Xu, X.-B.; Duan, S.-M.; Ren, L.; Shao, Y.-L.; Wang, Y.-W. Stereospecific synthesis of tetrahydronaphtho[2,3-b]furans enabled by a nickel-promoted tandem reductive cyclization. Org. Lett. 2016, 18, 5170–5173. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, C.; Zhang, Y.-Q.; Suo, S.-N.; Wang, Y.-W.; Peng, Y. A red-NIR fluorescence probe for rapid and visual detection of acrolein. Chem. Commun. 2022, 58, 10080–10083. [Google Scholar] [CrossRef]
- Feng, Y.-A.; Xu, H.; Zhou, Y.; Wang, B.-J.; Xiao, J.; Wang, Y.-W.; Peng, Y. Ratiometric detection and bioimaging of endogenous alkaline phosphatase by a NIR fluorescence probe. Sens. Actuators B 2022, 358, 131505. [Google Scholar] [CrossRef]
- Xu, H.; Wu, S.-L.; Lin, N.-J.; Lu, Y.; Xiao, J.; Wang, Y.-W.; Peng, Y. A NIR fluorescent probe for rapid turn-on detection and bioimaging of hypochlorite anion. Sens. Actuators, B. 2021, 346, 130484. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Y.-W.; Feng, W.; Peng, Y. Development of BINOL-Si complexes with large stokes shifts and their application as chemodosimeters for nerve agent. Chin. Chem. Lett. 2020, 31, 2960–2964. [Google Scholar] [CrossRef]
- Wang, B.-J.; Liu, R.-J.; Fang, J.; Wang, Y.-W.; Peng, Y. A water-soluble dual-site fluorescent probe for the rapid detection of cysteine with high sensitivity and specificity. Chem. Commun. 2019, 55, 11762–11765. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.S.; Rychnovsky, S.D. Total synthesis and structure revision of (−)-illisimonin A, a neuroprotective sesquiterpenoid from the fruits of Illicium simonsii. J. Am. Chem. Soc. 2019, 141, 13295–13300. [Google Scholar] [CrossRef]
- Kraatz, H.-B.; Lusztyk, J.; Enright, G.D. Ferrocenoyl amino acids: A synthetic and structural study. Inorg. Chem. 1997, 36, 2400–2405. [Google Scholar] [CrossRef]
- CCDC-2225030 (1) contain the supplementary crystallographic data for this paper. This data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: + 44 1223 336033.
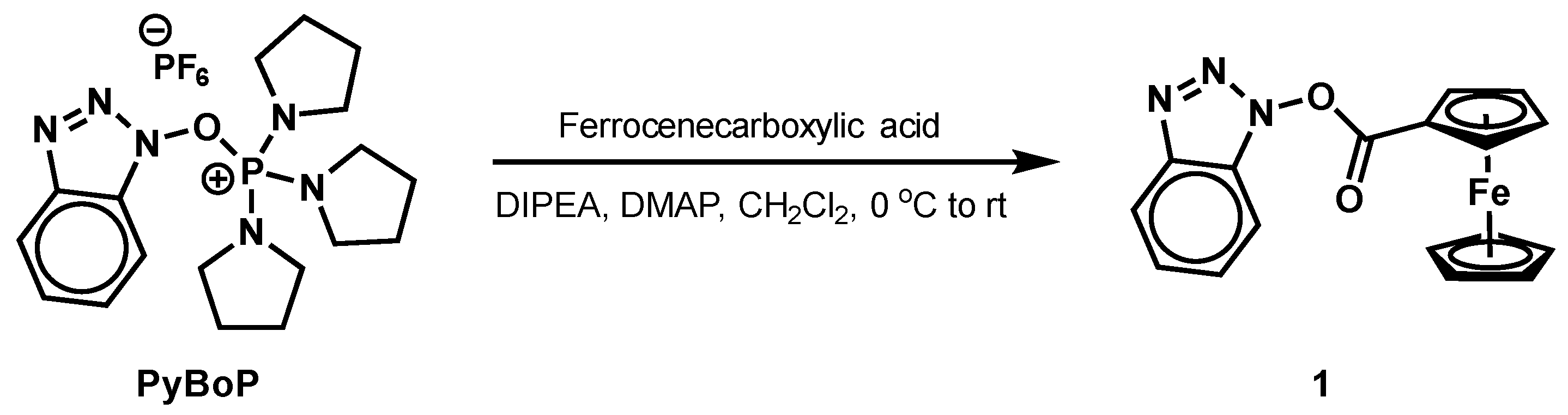
| Entry a | PyBoP (equiv.) | DIPEA (equiv.) | DMAP (equiv.) | PPY (equiv.) | 1 Yield b |
|---|---|---|---|---|---|
| 1 c | 1.0 | 1.8 | 0.6 | / | 23% |
| 2 | 1.0 | 1.8 | 0.6 | / | 25% |
| 3 d | 1.0 | 1.8 | 0.6 | / | 14% |
| 4 | 1.5 | 1.8 | 0.6 | / | 30% |
| 5 | 1.5 | 1.8 | / | / | 42% |
| 6 | 1.0 | 3.6 | 0.6 | / | 9% |
| 7 | 1.0 | 1.8 | 1.5 | / | 6% |
| 8 | 1.0 | 1.8 | / | 0.6 | 35% |
| 9 | 1.5 | 0.9 | / | 0.2 | 52% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.-Y.; Xu, L.-J.; Wang, Y.-W.; Peng, Y. New Preparation of Ferrocene Carboxylic Acid Benzotriazol-1-yl Ester. Molbank 2023, 2023, M1582. https://doi.org/10.3390/M1582
Zhang L-Y, Xu L-J, Wang Y-W, Peng Y. New Preparation of Ferrocene Carboxylic Acid Benzotriazol-1-yl Ester. Molbank. 2023; 2023(1):M1582. https://doi.org/10.3390/M1582
Chicago/Turabian StyleZhang, Lin-Yuan, Li-Jun Xu, Ya-Wen Wang, and Yu Peng. 2023. "New Preparation of Ferrocene Carboxylic Acid Benzotriazol-1-yl Ester" Molbank 2023, no. 1: M1582. https://doi.org/10.3390/M1582
APA StyleZhang, L.-Y., Xu, L.-J., Wang, Y.-W., & Peng, Y. (2023). New Preparation of Ferrocene Carboxylic Acid Benzotriazol-1-yl Ester. Molbank, 2023(1), M1582. https://doi.org/10.3390/M1582








