Abstract
Thiazolopyrimidines are attractive to medical chemists as new antitumor agents due to their high inhibitory activity against the replication process of tumor cells and the easy modification of their structure by changing the number and nature of substituents. The presence of asymmetric C5 carbon atoms requires the development of racemic mixture separation procedures for these heterocycles. One of the most effective methods is the crystallization of a racemic compound in the form of a conglomerate. The prerequisite for such separation is the construction of chiral, supramolecular ensembles in the crystalline state. Halogen-π interactions were chosen as supramolecular synthons. In this context, ethyl 7-methyl-3-oxo-2,3-dihidro-5H-thiazolo[3,2-a]pyrimidine-6-carboxylate containing a 4-bromophenyl fragment at C5 was synthesized. The crystal structure of the resulting compound was established using SCXRD. The role of the halogen-π interaction on the formation of one-dimensional homochiral chains is revealed.
1. Introduction
Thiazolo[3,2-a]pyrimidines, especially those that are 2-substituted, are promising structural fragments for the development of drugs, including anti-cancer drugs, in addition to their huge synthetic potential[1,2,3]. The structure of thiazolo[3,2-a]pyrimidine is quite easily modified by the introduction of new binding centers, which is extremely necessary to optimize the interaction of the ligand with the active center of the biotarget [4]. The presence of an asymmetric carbon atom, C5, determines the existence of two enantiomers (R- and S-isomers). One of the methods of separation for racemic compounds is the method of crystallization with a chiral seed: the so-called method of crystallization by attraction [5]. To implement this approach, it is necessary to establish experimental conditions for the crystallization of a racemic compound in the form of a conglomerate, i.e., in the form of a mechanical mixture of crystals of individual enantiomers. The formation of non-covalent, supramolecular [6,7,8]—or, in this case, halogen—interactions can lead to the formation of such crystal structures.
There are various synthetic approaches for obtaining derivatives of thiazolo[3,2-a]pyrimidines (Scheme 1). The most common approach is the cyclization of 3,4-dihydropyrimidine-2-thions 1 using biselectrophilic building blocks, which are most often halogen-containing: α-bromoketones, chloroacetic acid, 1,2-dichloroethane, and terminal alkynes [9,10,11,12,13,14,15,16,17,18].
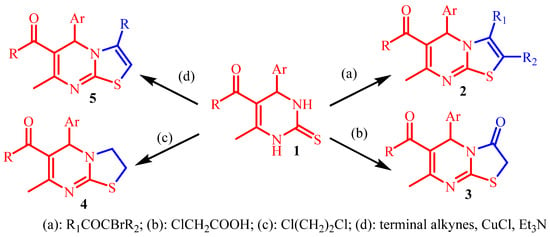
Scheme 1.
Synthetic approaches for obtaining derivatives of thiazolo[3,2-a]pyrimidines.
2. Results and Discussion
The target compound 7 was obtained with a high yield (90%) by a sequence of two reactions: three-component Biginelli condensation and the condensation of the resulting 1,2,3,4-tetrahydropyrimidine-2-thion 6 with ethyl chloroacetate. The initial condensation of acetoacetic ether, thiourea, and 4-brombenzaldehyde was carried out without a solvent at 120 °C. The subsequent condensation was also carried out in the absence of a solvent at 120 °C in a 5-fold excess of an alkylating agent (Scheme 2) [19,20,21].
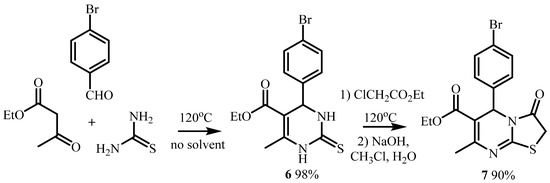
Scheme 2.
Synthesis of ethyl 5-(4-bromophenyl)-7-methyl-3-oxo-2,3-dihidro-5H-thiazolo[3,2-a]pyrimidine-6-carboxylate.
The structure of compound 7 was unambiguously confirmed using 1H and 13C NMR spectroscopy (see Figures S1 and S2), ESI mass spectrometry (see Figure S3), and single-crystal X-ray diffraction (Table S1). According to a single-crystal XRD data analysis, the bicycles tiazolo[3,2-a]pyrimidine fragment is found almost flat (Figure 1a). A six-membered cycle adopts the sofa conformation; the sp3 carbon atom C5 deviates slightly from the plane formed by other five atoms. The ethoxycarbonyl substituent is lying also in the plane of the bicyclic fragment. In crystal, the formation of n-π bonding is observed involving the Br-atom belonging to phenyl substituent at the C5 atom and C13 atom (dBr1…C13 = 3.379(1) Å, φ = 145.21(4)°). It is interesting to note that the established n-π interaction leads to the formation of homochiral chains of in the crystalline phase (Figure 1b). Homochiral chains consisting of one particular R- or S-isomer due to π-stacking are arranged parallel to each other (Figure 1c). Thus, it was discovered that the n-π interactions between the brom atom and the carbon atom of the phenyl substituent determined the crystal packing of 7.
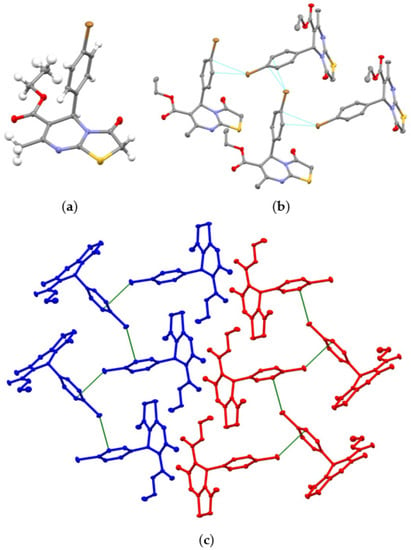
Figure 1.
ORTEP view of molecule 7 in the crystalline phase (a) (Br, C, O, N, S, and H-atoms are presented as brown, grey, red, light-violet, yellow, and light grey ellipsoids with a 50% probability, respectively), a mutual orientation of R-isomers of 7 within the zigzag homochiral chain formed by Br1…C13 n-π interaction (b), and a portion of crystal packing of 7 showing the formation of parallel R- and S-homochiral chains along b axis (colored in blue and red, respectively) (c) (H-atoms are omitted for clarity).
3. Materials and Methods
NMR experiments were performed on Bruker Avance instruments with an operating frequency of 500 MHz for shooting 1H and 13C NMR spectra. Chemical shifts were determined relative to the signals of residual protons of the CDCl3. Electrospray ionization (ESI) mass spectra were obtained using a Bruker AmaZon X ion trap mass spectrometer. IR spectra in KBr tablets were recorded on a Bruker Vector-22.
Ethyl 4-(4-bromophenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate was synthesized.
Acetoacetic ether (1 mmol), 4-brombenzaldehyde (1 mmol), and thiourea (1.5 mmol) were fused at 120 °C without solvent for 8 h. Organic residue was recrystallized from ethanol (30 mL). The precipitate was filtered, washed with a small amount of methanol (5 mL), and dried in air. The data from the 1H and 13C NMR spectroscopy of the obtained tetrahydropyrimidine-2-thion coincided with the literature [22].
Ethyl 5-(4-bromophenyl)-7-methyl-3-oxo-2,3-dihydro-5H-thiazolo[3,2-a]pyrimidine-6-carboxylate 7 was synthesized.
1,2,3,4-Tetrahydripyrimidine-2-thion 6 (1 mmol) was mixed with ethyl chloroacetate (5 mmol) without a solvent. The mixture was stirred at a temperature of 120 °C for 1 h. It was then cooled to room temperature, and ethyl acetate (20 mL) was added and filtered out. The precipitate was washed with ethyl acetate and recrystallized from ethyl alcohol. To obtain thiazolo[3,2-a]pyrimidine 7 as a base, 10 mL of chloroform and 10 mL of 1M aqueous NaOH solution were added to 1 mmol of the formed hydrochloride and stirred at room temperature for 1 h. The organic part was then separated, dried over anhydrous sodium sulfate, and evaporated on rotary evaporators affording a light orange oil. TLC Rf = 0.4 (CHCl3 with 1% EtOH)
1H NMR (500 MHz, DMSO-d6, 25 °C) δH ppm: 1.11 (t, J = 7.1 Hz, 3H, OCH2CH3), 2.35 (s, 3H, CH3), 4.00–4.03 (m, 2H, OCH2CH3), 4.12 (d, J = 4 Hz, 2H, CH2), 5.86 (s, 1H, CH-Ar), 7.20–7.22(d, J = 8.4 Hz, 2H, CH (Ar)), 7.54–7.56 (d, J = 8.5 Hz, 2H, CH (Ar)). 13C NMR (100 MHz, DMSO-d6, 25 °C) δC ppm: 14.36, 22.95, 33.04, 54.59, 60.53, 107.17, 122.10, 130.15, 132.04, 140.44, 152.52, 161.59, 165.28, 171.51. IR (KBr, cm−1): 2977 (CH2); 1741 (C=O); 1709 (C=O); 1619; 1536; 1237; 836; 752. MS (ESI), m/z, [M+H]+: calcd. for C16H16BrN2O3S+: 396,28; found: 397,07. Anal. Calcd. for C16H15BrN2O3S, %: C 48.62, H 3.83, Br 20.21; N 7.09; O 12.14, S 8.11. Found C 48.45, H 3.76, Br 20.47; N 7.18; O 12.26, S 7.88.
Crystals of 7 suitable for X-ray diffraction study were obtained by the slow evaporation of a tetrahydrofuran solution (20 mL) containing 0.02 mol of dissolved compound after 2 days.
An X-ray diffraction analysis of 7 was performed on a Bruker D8 QUEST automatic three-circle diffractometer with a PHOTON III two-dimensional detector and an IμS DIAMOND microfocus X-ray tube (λ[Mo Kα] = 0.71073 Å) at cooling conditions. Data collection and the processing of diffraction data were performed using APEX3 software package.
Structure 7 was solved by the direct method using the SHELXT program [23]. It was refined by the full-matrix least squares method over F2 using the SHELXL program [24]. All calculations were performed in the WinGX software package [25]. The calculations of the geometry of molecules and intermolecular interactions in crystals were carried out using the PLATON program [26]. The drawings of molecules were performed using the OR-TEP-3 [25] and MERCURY [27] programs.
Non-hydrogen atoms were refined in the anisotropic approximation. The positions of the hydrogen atoms H(O) were determined using difference Fourier maps, and these atoms were refined isotropically. The remaining hydrogen atoms were placed in geometrically calculated positions and included in the refinement in the “riding” model. Crystallographic data of structure 7 was deposited at the Cambridge Crystallographic Data Center; registration numbers and the most important characteristics are provided in Tables S1–S4.
4. Conclusions
In this work, a new racemic ethyl 5-(4-bromophenyl)-7-methyl-3-oxo-2,3-dihydro-5H-thiazolo[3,2-a]pyrimidine-6-carboxylate (7) was obtained and characterized, including by single crystal X-ray diffraction (SCXRD). It was shown that the generation of one-dimensional, homochiral, supramolecular assembly in the crystalline phase of 7 can be achieved due to weak n-π interactions involving the halogen Br-atom of a phenyl substituent at the C5 atom. Thus, this work can be considered the first step in searching for the proper conditions for the chiral separation of thiazolo[3,2-a]pyrimidine derivatives—potentional anti-tumor agents—via chiral discrimination.
Supplementary Materials
The following supporting information can be downloaded. Figure S1: 1H NMR spectrum of compound 7 (DMSO-d6, 500 MHz, 25 °C); Figure S2: 13C NMR spectrum of compound 7 (DMSO-d6, 100 MHz, 25 °C); Figure S3: ESI MS spectrum of compound 7 (Ion Polarity: Positive); Table S1: Crystallographic data for compound 7; Table S2: Bond distances and angles of asymmetric C5 atom in studied compounds, established by SCXRD for 7; Table S3: Selected bond distances and dihedral angles for studied compounds 7 established by SCXRD; Table S4: The distances between the bromine atom and the 4-bromophenyl fragment present in the crystals of the studied compound.
Author Contributions
Conceptualization, A.A., I.A. and S.S.; methodology, A.O. and I.L.; validation, A.A. and I.L.; formal analysis, A.A.; investigation, A.N., A.A. and I.L.; resources, I.L.; data curation, A.A., I.A. and S.S.; writing—original draft preparation, A.A.; writing—review and editing, A.A.; visualization, A.A.; supervision, I.A. and S.S.; project administration, A.A., I.A. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by financial support from the government assignment for the Arbuzov Institute of Organic and Physical Chemistry, FRC Kazan Scientific Center, Russian Academy of Sciences (122011800132-5).
Data Availability Statement
The data presented in this study are contained within the article or in Supplementary Materials, or are available on request from the corresponding author Artem Agarkov.
Acknowledgments
The authors are grateful to the Assigned Spectral-Analytical Center of Shared Facilities for Study of Structure, Composition and Properties of Substances and Materials of the Federal Research Center of Kazan Scientific Center of Russian Academy of Sciences (CSF-SAC FRC KSC RAS) for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Keshari, A.K.; Singh, A.K.; Saha, S. Bridgehead Nitrogen Thiazolo[3,2-a]pyrimidine: A Privileged Structural Framework in Drug Discovery. Mini-Rev. Med. Chem. 2017, 17, 1488–1499. [Google Scholar] [CrossRef]
- Kashyap, S.J.; Sharma, P.K.; Garg, V.K.; Dudhe, R.; Kumar, N. Review on Synthesis and Various Biological Potential of Thiazolopyrimidine Derivatives. J. Adv. Sci. Res. 2011, 2, 18–24. [Google Scholar]
- Agarkov, A.S.; Nefedova, A.A.; Gabitova, E.R.; Mingazhetdinova, D.O.; Ovsyannikov, A.S.; Islamov, D.R.; Amerhanova, S.K.; Lyubina, A.P.; Voloshina, A.D.; Litvinov, I.A.; et al. (2-Hydroxy-3-Methoxybenzylidene)thiazolo[3,2-a]pyrimidines: Synthesis, Self-Assembly in the Crystalline Phase and Cytotoxic Activity. Int. J. Mol. Sci. 2023, 24, 2084. [Google Scholar] [CrossRef]
- Chen, L.; Jin, Y.; Fu, W.; Xiao, S.; Feng, C.; Fang, B.; Gu, Y.; Li, C.; Zhao, Y.; Liu, Z.; et al. Design, Synthesis, and Structure–Activity Relationship Analysis of Thiazolo[3,2-a]pyrimidine Derivatives with Anti-inflammatory Activity in Acute Lung Injury. ChemMedChem 2017, 12, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Bredikhin, A.A.; Savel”ev, D.V.; Bredikhina, Z.A.; Gubaidullin, A.T.; Litvinov, I.A. Crystallization of chiral compounds. 2. Propranolol: Free base and hydrochloride. Russ. Chem. Bull. 2003, 52, 853–861. [Google Scholar] [CrossRef]
- Agarkov, A.S.; Gabitova, E.R.; Galieva, F.B.; Ovsyannikov, A.S.; Voloshina, A.D.; Shiryaev, A.K.; Litvinov, I.A.; Solovieva, S.E.; Antipin, I.S. Structure and Biological Properties of 2-Phenylhydrazone Derivatives of Thiazolopyrimidines. Dokl. Chem. 2022, 503, 45–50. [Google Scholar] [CrossRef]
- Agarkov, A.S.; Litvinov, I.A.; Gabitova, E.R.; Ovsyannikov, A.S.; Dorovatovskii, P.V.; Shiryaev, A.K.; Solovieva, S.E.; Antipin, I.S. Crystalline State Hydrogen Bonding of 2-(2-Hydroxybenzylidene)Thiazolo[3,2-a]Pyrimidines: A Way to Non-Centrosymmetric Crystals. Crystals 2022, 12, 494. [Google Scholar] [CrossRef]
- Agarkov, A.S.; Nefedova, A.A.; Gabitova, E.R.; Ovsyannikov, A.S.; Amerhanova, S.K.; Lyubina, A.P.; Voloshina, A.D.; Dorovatovskii, P.V.; Litvinov, I.A.; Solovieva, S.E.; et al. Synthesis, Self-Assembly in Crystalline Phase and Anti-Tumor Activity of 2-(2-/4-Hydroxybenzylidene)thiazolo[3,2-a]pyrimidines. Molecules 2022, 27, 7747. [Google Scholar] [CrossRef]
- Zhi, H.; Chen, L.-M.; Zhang, L.-L.; Liu, S.-J.; Wan, D.C.C.; Lin, H.-Q.; Hu, C. Design, synthesis, and biological evaluation of 5H-thiazolo[3,2-a]pyrimidine derivatives as a new type of acetylcholinesterase inhibitors. ARKIVOC 2008, 13, 266. [Google Scholar] [CrossRef]
- Quan, Z.-J.; Zhang, Z.; Wang, J.-K.; Wang, X.-C.; Liu, Y.-J.; Ji, P.-Y. Efficient synthesis of 5H-thiazolo[3,2-a]pyrimidines from reactions of 3,4-dihydropyrimidine-thiones with α-bromoacetone in aqueous media. Heteroat. Chem. 2008, 2, 149. [Google Scholar] [CrossRef]
- Wichmann, J.; Adam, G.; Kolczewski, S.; Mutel, V.; Woltering, T. Structure-activity relationships of substituted 5H-thiazolo[3,2-a]pyrimidines as group 2 metabotropic glutamate receptor antagonists. Bioorg. Med. Chem. Lett. 1999, 9, 1573. [Google Scholar] [CrossRef]
- Danel, K.; Pedersen, E.B.; Nielsen, C.J. Synthesis and Anti-HIV-1 Activity of Novel 2,3-Dihydro-7H-thiazolo[3,2-a]pyrimidin-7-ones. Med. Chem. 1998, 41, 191. [Google Scholar] [CrossRef]
- Pan, B.; Huang, R.; Zheng, L.; Chen, C.; Han, S.; Qub, D.; Zhu, M.; Wei, P. Thiazolidione derivatives as novel antibiofilm agents: Design, synthesis, biological evaluation, and structure–activity relationships. Eur. J. Med. Chem. 2011, 46, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.F.; Flefel, E.M.; Amr, A.E.; Abd El-Shafy, D.N. Anti-HSV-1 activity and mechanism of action of some new synthesized substituted pyrimidine, thiopyrimidine and thiazolopyrimidine derivatives. Eur. J. Med. Chem. 2012, 45, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.E.; Sayed, H.H.; Shamroukh, A.H.; Awad, H.M. Preparation of Some Fused Pyridopyrimidine and Pyridothienotriazine Derivatives for Biological Evaluation. Phosphorus Sulfur Silicon 2005, 180, 2767. [Google Scholar] [CrossRef]
- Kolb, S.; Mondesert, O.; Goddard, M.-L.; Jullien, D.; Villoutreix, B.O.; Ducommun, B.; Garbay, C.; Braud, E. Development of Novel Thiazolopyrimidines as CDC25B Phosphatase Inhibitors. Chem. Med. Chem. 2009, 4, 633–648. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Mohamad, Y.A.; Mohamed, S.A.; Ammar, Y.A. Synthesis and radiation stability of novel thiazolopyrimidines with expected antifungal activity. Phosphorus Sulfur Silicon Relat. Elem. 1996, 108, 249–256. [Google Scholar] [CrossRef]
- Agarkov, A.S.; Kozhikhov, A.A.; Nefedova, A.A.; Ovsyannikov, A.S.; Islamov, D.R.; Solovieva, S.E.; Antipin, I.S. New Method for the Preparation of 2,3-Disubstituted 2,3-Dihydrothiazolo[3,2-a]pyrimidines. Dokl. Chem. 2022, 505, 177–183. [Google Scholar] [CrossRef]
- Shiryaev, A.K.; Baranovskaya, N.S.; Eremin, M.S. Synthesis of 5H-thiazolo [3, 2-a] pyrimidines. Chem. Heterocycl. Compd. 2013, 48, 1550–1554. [Google Scholar] [CrossRef]
- Shiryaev, A.K.; Kolesnikova, N.G.; Kuznetsova, N.M.; Lashmanova, E.A. Alkylation of tetrahydropyrimidine-2-thions with ethyl chloroacetate. Chem. Heterocycl. Compd. 2013, 49, 1812–1817. [Google Scholar]
- Lashmanova, E.A.; Shiryaev, A.K. Nitrosation of 5 H-thiazolo [3, 2-a] pyrimidin-3 (2 H)-ones. Chem. Heterocycl. Compd. 2015, 51, 377–380. [Google Scholar] [CrossRef]
- Shaibuna, M.; Kuniyil, M.J.K.; Sreekumar, K. Deep eutectic solvent assisted synthesis of dihydropyrimidinones/thiones via Biginelli reaction: Theoretical investigations on their electronic and global reactivity descriptors. New J. Chem. 2021, 45, 20765–20775. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Streek, J.V.D. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).