6-Chloro-3-nitro-2-[(phenylsulfonyl)methyl]imidazo[1,2-b]pyridazine
Abstract
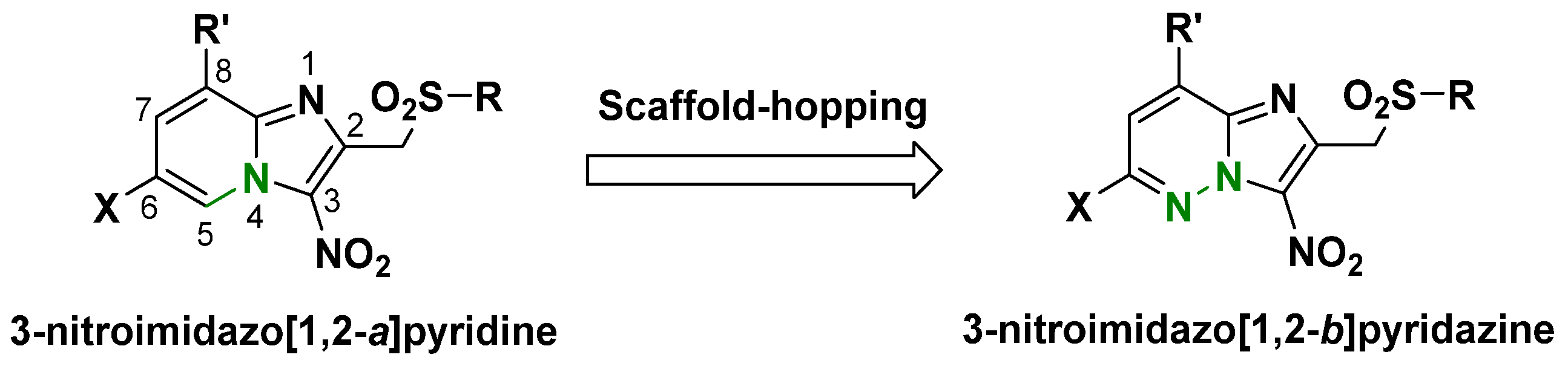
:1. Introduction
2. Results and Discussion
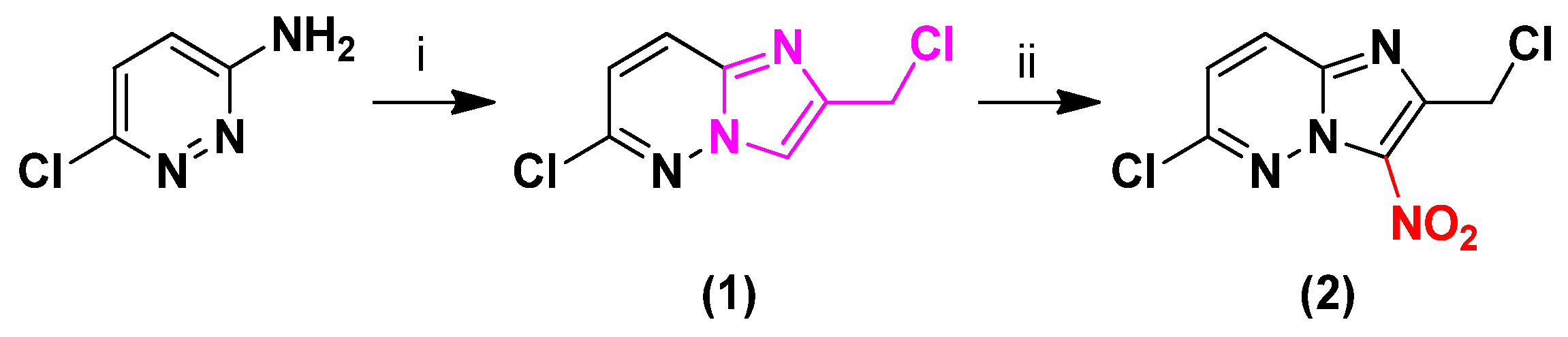
2.1. Synthesis
2.2. Biological Results
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. 6-Chloro-2-(chloromethyl)imidazo[1,2-b]pyridazine (1)
3.1.3. 6-Chloro-2-(chloromethyl)-3-nitroimidazo[1,2-b]pyridazine (2)
3.1.4. 6-Chloro-3-nitro-2-[(phenylsulfonyl)methyl]imidazo[1,2-b]pyridazine (3)
3.2. Biology
3.2.1. Antileishmanial Activity against L. donovani Promastigotes
3.2.2. Antileishmanial Activity on L. infantum Axenic Amastigotes
3.2.3. Antitrypanosomal Evaluation on T. b. brucei BSF Trypomastigotes
3.2.4. Cytotoxicity Evaluation on HepG2 Cell Line
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, B.; Gupta, N. Taming Parasites by Tailoring Them. Front. Cell. Infect. Microbiol. 2017, 7, 292. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, F.; Gradoni, L. (Eds.) The Leishmaniases: Old Neglected Tropical Diseases; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-72385-3. [Google Scholar]
- World Health Organization (WHO). Trypanosomiasis, Human African (Sleeping Sickness). Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 13 January 2023).
- World Health Organization (WHO). Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 13 January 2022).
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Simarro, P.P.; Cecchi, G.; Franco, J.R.; Paone, M.; Diarra, A.; Ruiz-Postigo, J.A.; Fèvre, E.M.; Mattioli, R.C.; Jannin, J.G. Estimating and Mapping the Population at Risk of Sleeping Sickness. PLoS Negl. Trop. Dis. 2012, 6, e1859. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis. Available online: https://apps.who.int/iris/handle/10665/77472 (accessed on 13 January 2023).
- Rao, S.P.S.; Barrett, M.P.; Dranoff, G.; Faraday, C.J.; Gimpelewicz, C.R.; Hailu, A.; Jones, C.L.; Kelly, J.M.; Lazdins-Helds, J.K.; Mäser, P.; et al. Drug Discovery for Kinetoplastid Diseases: Future Directions. ACS Infect. Dis. 2019, 5, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Fersing, C.; Basmaciyan, L.; Boudot, C.; Pedron, J.; Hutter, S.; Cohen, A.; Castera-Ducros, C.; Primas, N.; Laget, M.; Casanova, M.; et al. Nongenotoxic 3-Nitroimidazo[1,2-a]Pyridines Are NTR1 Substrates That Display Potent in Vitro Antileishmanial Activity. ACS Med. Chem. Lett. 2019, 10, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Böhm, H.-J.; Flohr, A.; Stahl, M. Scaffold Hopping. Drug Discov. Today Technol. 2004, 1, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Terme, T.; Maldonado, J.; Crozet, M.P.; Vanelle, P.; Galtier, C.; Gueiffier, A. Synthesis of 2-Substituted-3-Nitroimidazo[1,2-b]Pyridazines as Potential Biologically Active Agents. J. Heterocycl. Chem. 2002, 39, 173–177. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Räz, B.; Iten, M.; Grether-Bühler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue® Assay to Determine Drug Sensitivity of African Trypanosomes (T.b. rhodesiense and T.b. gambiense) in Vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Baltz, T.; Baltz, D.; Giroud, C.; Crockett, J. Cultivation in a Semi-Defined Medium of Animal Infective Forms of Trypanosoma Brucei, T. Equiperdum, T. Evansi, T. Rhodesiense and T. Gambiense. EMBO J. 1985, 4, 1273–1277. [Google Scholar] [CrossRef] [PubMed]

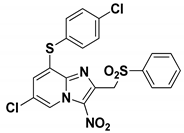 | |
| Hit A | |
| EC50 L. donovani promastigotes (µM) | 1.0 ± 0.3 |
| EC50 L. infantum axenic amastigotes (µM) | 1.7 ± 0.3 |
| EC50 T. b. brucei BSF (µM) | 0.95 ± 0.05 |
| CC50 HepG2 (µM) | >100 |
| CC50 HepG2 (µM) | EC50 L. dono. pro. (µM) | EC50 L. inf. Axenic Ama. (µM) | EC50 T. b. brucei BSF (µM) | |
|---|---|---|---|---|
| 3 | >7.8 a | >15.6 a | >1.6 a | 0.38 ± 0.03 |
| Hit A | >100 | 1.0 ± 0.3 | 1.7 ± 0.3 | 0.95 ± 0.05 |
| Doxorubicin b | 0.20 ± 0.02 | - | - | - |
| Amphotericin B c | 8.8 ± 0.3 | 0.07 ± 0.01 | 0.08 ± 0.02 | - |
| Miltefosine c | 85.0 ± 8.8 | 3.1 ± 0.2 | 0.30 ± 0.02 | - |
| Fexinidazole c,d | >100 | 1.2 ± 0.2 | 7.8 ± 2.3 | 1.4 ± 0.5 |
| Suramin d | >100 | - | - | 0.08 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paoli-Lombardo, R.; Primas, N.; Hutter, S.; Bourgeade-Delmas, S.; Boudot, C.; Castera-Ducros, C.; Jacquet, I.; Courtioux, B.; Azas, N.; Rathelot, P.; et al. 6-Chloro-3-nitro-2-[(phenylsulfonyl)methyl]imidazo[1,2-b]pyridazine. Molbank 2023, 2023, M1573. https://doi.org/10.3390/M1573
Paoli-Lombardo R, Primas N, Hutter S, Bourgeade-Delmas S, Boudot C, Castera-Ducros C, Jacquet I, Courtioux B, Azas N, Rathelot P, et al. 6-Chloro-3-nitro-2-[(phenylsulfonyl)methyl]imidazo[1,2-b]pyridazine. Molbank. 2023; 2023(1):M1573. https://doi.org/10.3390/M1573
Chicago/Turabian StylePaoli-Lombardo, Romain, Nicolas Primas, Sébastien Hutter, Sandra Bourgeade-Delmas, Clotilde Boudot, Caroline Castera-Ducros, Inès Jacquet, Bertrand Courtioux, Nadine Azas, Pascal Rathelot, and et al. 2023. "6-Chloro-3-nitro-2-[(phenylsulfonyl)methyl]imidazo[1,2-b]pyridazine" Molbank 2023, no. 1: M1573. https://doi.org/10.3390/M1573
APA StylePaoli-Lombardo, R., Primas, N., Hutter, S., Bourgeade-Delmas, S., Boudot, C., Castera-Ducros, C., Jacquet, I., Courtioux, B., Azas, N., Rathelot, P., & Vanelle, P. (2023). 6-Chloro-3-nitro-2-[(phenylsulfonyl)methyl]imidazo[1,2-b]pyridazine. Molbank, 2023(1), M1573. https://doi.org/10.3390/M1573









