Abstract
The solvent-free Betti reaction of 2-naphthol, 4-nitrobenzaldehyde and (S)-valine methyl ester gave the corresponding aminobenzylnaphthol of the (S,S)-2-(((hydroxynaphth-1-yl)(4′-nitrophenyl)methyl)amino)-3-methylbutanoic acid methyl ester in good yield (59%). This product was fully characterized. We observed that the racemization that occurs in some Betti reactions with (S)-valine methyl ester was absent in this reaction, and thus the predominant (S,S)-product was obtained.
1. Introduction
The Betti reaction is a straightforward condensation that assembles 2-naphthol, aryl aldehydes and amines to yield aminobenzylnaphthol [1,2,3]. It was reported by Mario Betti at the beginning of the 20th century [3] and rarely used, until our rediscovery in the recent years of the same century [1,2].
After our first reports, many researchers used this procedure to synthesize aminobenzylnaphthols [4]. Starting from 2019, a new interest has grown in the investigation of Betti compounds, especially as bioactive compounds [5].
Our contribution to this topic includes an evaluation of the action of these molecules towards Candida albicans [6], and later an evaluation of antiproliferative activity towards two tumor cell lines [7]. In both papers, Betti aminobenzylnaphthols encompassing an amino acid residue were investigated.
2. Results and Discussion
Incorporating an amino acid moiety into a Betti aminobenzylnaphthol is not an easy task [8,9].
When an enantiopure amine is employed in the Betti reaction, in principle, two diastereoisomers are obtained (Scheme 1).
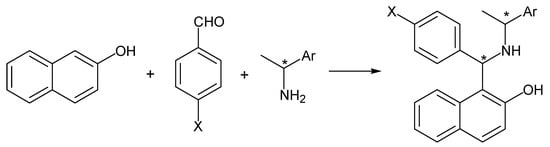
Scheme 1.
Betti reaction between 2-naphthol, aryl aldehydes and (S)- or (R)-1-arylethylamines.
For example, (S,S)- and (R,S)-stereoisomers are produced in the Betti reaction involving (S)-1-arylethylamine, aryl aldehydes and 2-naphthol [10]. Suitable reaction conditions can improve this stereochemical pathway with the predominant formation of the (S,S)-stereoisomer, which can be easily obtained free from the (R,S)-counterpart [10].
On the other hand, an extensive scrambling of the stereogenic center could affect Betti reactions in which (S)-aminoacid methyl esters are employed as amines [8]. All of the four possible aminobenzylnaphthols (i.e., (S,S)- (S,R)-, (R,S)- and (R,R)-stereoisomers) could be obtained [8].
This disappointing result can be explained by analyzing the reaction mechanism. In the Betti procedure, an imine between aryl aldehyde and the amine is formed in a preliminary step [3,8]. This imine undergoes the addition of 2-naphthol, which behaves as a carbon nucleophile [3,8].
An imine deriving from an amino acid methyl ester and an aryl aldehyde could be subjected to a tautomeric equilibrium (Scheme 2) that causes the complete scrambling of the original (S)-stereogenic center deriving from the amino acid [8].
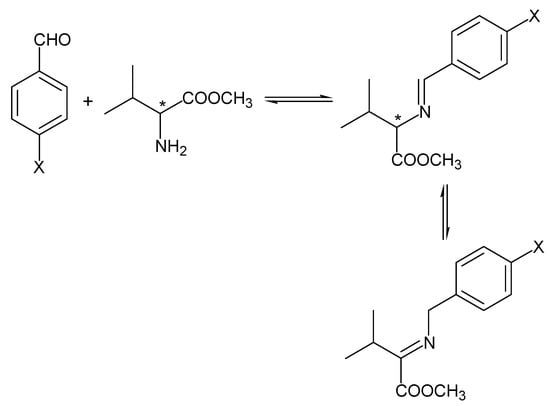
Scheme 2.
Tautomeric equilibrium of an imine.
Higher temperature and adequate substituents on the aryl groups favor this undesired reaction pathway [8]. For example, in the Betti reaction involving (S)-valine methyl ester and benzaldehyde, a 60 °C reaction temperature causes the complete scrambling of the stereogenic centers [8]. A different reaction set up (the reactants are mixed in diethyl ether at 35 °C in the presence of lithium perchlorate and chlorotrimethylsilane) is required in order to prevent racemization and to obtain only the (S,S)- and (R,S)-stereoisomers, with the (S,S)-stereoisomer being predominant and more easily isolable [8].
In our past screening of different aryl aldehydes, we tested 4-fluoro- and 4-chlorobenzaldehyde in reactions with 2-naphthol and (S)-valine methyl ester [8,9]. The desired (S,S)-aminobenzylnaphthols were obtained in good yield and without any particular stereochemical issue, even when higher temperatures (60 °C) were applied [8,9].
At this point, we found it interesting to test the Betti reaction with 2-naphthol, (S)-valine methyl ester and different aryl aldehydes to obtain more information on the stereochemistry of the process.
In the reaction involving (S)-valine methyl ester, 2-naphthol and 4-anisaldehyde, a loss of stereochemical control occurred in the usual reaction conditions (no solvent; 60 °C temperature). The application of milder reaction conditions (diethyl ether as a solvent; milder temperatures of 0–35 °C; the addition of lithium perchlorate and chlorotrimethylsilane as catalysts) was not able to fix this scrambling.
At this stage, we decided to abandon further attempts with this aldehyde and to try with an aryl aldehyde bearing a substituent of opposite electronic properties. In this paper, we report the results of our investigation on the reaction between 2-naphthol, (S)-valine methyl ester and 4-nitrobenzaldehyde (Scheme 3).
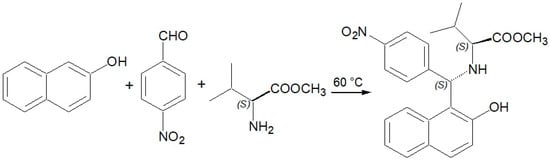
Scheme 3.
Betti reaction between 2-naphthol, 4-nitrobenzaldehyde and (S)-valine methyl ester.
This reaction was successful. We observed no racemization, even under harsh reaction conditions (60 °C, no solvent). The corresponding (S,S)-aminobenzylnaphthol was obtained in good yield (59%). In the crude reaction mixture, we recognized unreacted starting materials, together with smaller amounts of the (R,S)-stereoisomer (ratio between the (S,S)-stereoisomer and the (R,S) one = 3.6). The (R,S)-stereoisomer was not investigated further.
In the absence of a crystal suitable for the X-ray diffraction experiment [9,10], the (S,S) configuration can be attributed to H(1)-NMR considerations. This procedure was employed by us and was found to be reliable [6,8] when it was compared with the absolute configurations obtained via X-ray diffraction experiments [9,10].
In this type of molecule, the H(1)-NMR signal of the benzyl hydrogen atom of the (S,S)-stereoisomer moves upfield with respect to the same signal of the (R,S)-stereoisomer [6,8]. In the crude reaction mixture, we observed two signals for that hydrogen, the first lying at 5.68 ppm (predominant, and subsequently isolated), and the other one at 5.93 ppm. According to our previous considerations, the (S,S)-configuration can be attributed to the aminobenzylnaphthol having the benzyl hydrogen signal at 5.68 ppm, thus confirming the trend in which every time we undertake a Betti reaction with an (S)-amine, (S,S)-aminobenzylnaphthols are regularly obtained [6,8,9,10].
This nitro-substituted compound will be evaluated for its bio-activity, as previously reported for the unsubstituted derivative [7].
3. Materials and Methods
Chemicals were used as received. NMR spectra were recorded on a Bruker AM500 spectrometer. MS spectra were obtained with an Agilent HPLC QTOF spectrometer via direct infusion of the samples. HPLC analyses were performed on an Agilent 1100 chromatograph equipped with a DAD detector.
(S)-Valine methyl ester (0.2 g, 1.52 mmol) was added to 4-nitrobenzaldehyde (0.2 g, 1.32 mmol) and stirred for 10 min at room temperature. 2-Naphthol (0.18 g, 1.25 mmol) was added, and the mixture was heated to 60 °C for two days. The crude reaction mixture was purified first by chromatography (silica gel, eluent n-hexane/ethyl acetate 9:1), followed by crystallization (ethanol) to yield 0.3 g (59%) of the (S,S)-stereoisomer free from the (S,R)-counterpart. mp 117–119 °C (ethanol), [α]D25 = + 98.4 (c = 1, CHCl3).
The enantiomeric purity of the obtained aminobenzylnaphthol was checked with chiral HPLC. In a first step, an (S,S)-+ (R,R)-mixture of the title compound was obtained by repeating this reaction with a mixture of (S)+ (R)-valine methyl ester. Then, this mixture was employed in the setting up of the separation conditions with chiral HPLC (column Chiralcel OD-H, eluent n-hexane/i-propanol 9:1; flow rate 0.5 mL/min; tSS = 14.9; tRR = 20.5; separation factor α = 1.54). Finally, the aminobenzylnaphthol obtained in the reaction with (S)-valine methyl ester was checked with HPLC in the established separation conditions to find the single (S,S)-stereoisomer.
1H-NMR (CDCl3, 500 MHz) δ 12.19–12.16 (broad, 1 H, OH), 8.18–8.14 (m, 2 H, Har), 7.79–7.75 (m, 2 H, HAr), 7.65–7.61 (m, 2 H, HAr), 7.56–7.52 (m, 1 H, HAr), 7.38–7.33 (m, 1 H, HAr), 7.30–7.26 (m, 1 H, HAr), 7.18 (d, J = 8.8 Hz, 1 H, HAr), 5.68 (broad, 1 H, HCAr2), 3.80 (s, 3 H, OCH3), 3.38 (dd, J = 5.1, J = 12.8 Hz, 1 H, HCC=O), 2.68 (d, J = 12.8 Hz, 1 H, NH), 2.12 (d septet, J = 5.1, J = 6.8 Hz, 1 H, CHMe2), 1.00 (d, J = 6.8 Hz, 3 H, CH3), 0.99 (d, J = 6.8 Hz, 3 H, CH3).
13C-NMR (CDCl3, 125 MHz) δ 174.4 (C=O), 156.5 (C-OH), 147.5 (CAr), 132.6 (CAr), 130.7 (CAr), 129.1 (CAr), 129.0 (CAr), 128.8 (CAr), 127.0 (CAr), 124.3 (CAr), 122.9 (CAr), 120.3 (CAr), 120.0 (CAr), 111.4 (CAr), 65.2 (C-C=O), 60.6 (HCAr2), 52.1 (OCH3), 31.6 (CHMe2), 19.0 (Me), 18.7 (Me).
HRMS (ESI-TOF), m/z: calcd for C23H24N2O5Na 431.1583, found [M + Na]+ 431.1527.
Graphical representations of the NMR and HRMS spectra and the HPLC profiles are available in the Supplementary Materials.
Supplementary Materials
The following supporting information can be downloaded. Spectral properties and HPLC profiles of the title compound.
Author Contributions
M.A.M.C. and C.C. contributed in the same way to all the steps of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The project Fibre e tessuti intelligenti ed ECOsostenibili per l’abbigliamento TECnico e l’alta moda (ECOTEC) is gratefully acknowledged for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Cardellicchio, C.; Ciccarella, G.F.; Schingaro, E.; Scordari, F. The Betti base: Absolute configuration and routes to a family of related chiral nonracemic bases. Tetrahedron Asymmetry 1998, 9, 3667–3675. [Google Scholar] [CrossRef]
- Cardellicchio, C.; Ciccarella, G.; Naso, F.; Perna, F.; Tortorella, P. Use of readily available chiral compounds related to the Betti base in the enantioselective addition of diethylzinc to aryl aldehydes. Tetrahedron 1999, 55, 14685–14692. [Google Scholar] [CrossRef]
- Naso, F. Mario Betti: A Giant in the Chemistry Scenario of the Twentieth Century. Substantia 2017, 1, 111–121. [Google Scholar]
- Cardellicchio, C.; Capozzi, M.A.M.; Naso, F. The Betti base: The awakening of a sleeping beauty. Tetrahedron Asymmetry 2010, 21, 507–517. [Google Scholar] [CrossRef]
- Iftikhar, R.; Kamran, M.; Iftikhar, A.; Parveen, S.; Naeem, N.; Jamil, N. Recent Advances in the green synthesis of Betti bases and their application: A review. Mol. Divers. 2022. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, M.A.M.; Cardellicchio, C.; Magaletti, A.; Bevilacqua, A.; Perricone, M.; Corbo, M.R. Bioactivity of a Family of Chiral Nonracemic Aminobenzylnaphthols towards Candida albicans. Molecules 2014, 19, 5219–5230. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, R.; Capozzi, M.A.M.; Cardellicchio, C. Antiproliferative Activity of Aminobenzylnaphthols Deriving from the Betti Reaction. Appl. Sci. 2022, 12, 7779. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Cardellicchio, C. Stereoselection in the Betti reaction of valine methyl esters. Tetrahedron Asymmetry 2017, 28, 1792–1796. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Alvarez-Larena, A.; Piniella Febrer, J.F.; Cardellicchio, C. A combined structural and computational investigation of aminobenzylnaphthol compounds derived from the Betti reaction using valine methyl ester. New J. Chem. 2021, 45, 20735–20742. [Google Scholar] [CrossRef]
- Cardellicchio, C.; Capozzi, M.A.M.; Alvarez-Larena, A.; Piniella, J.F.; Capitelli, F. Investigation on the weak interactions assembling the crystal structures of Betti bases. CrystEngComm 2012, 14, 3972–3981. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).