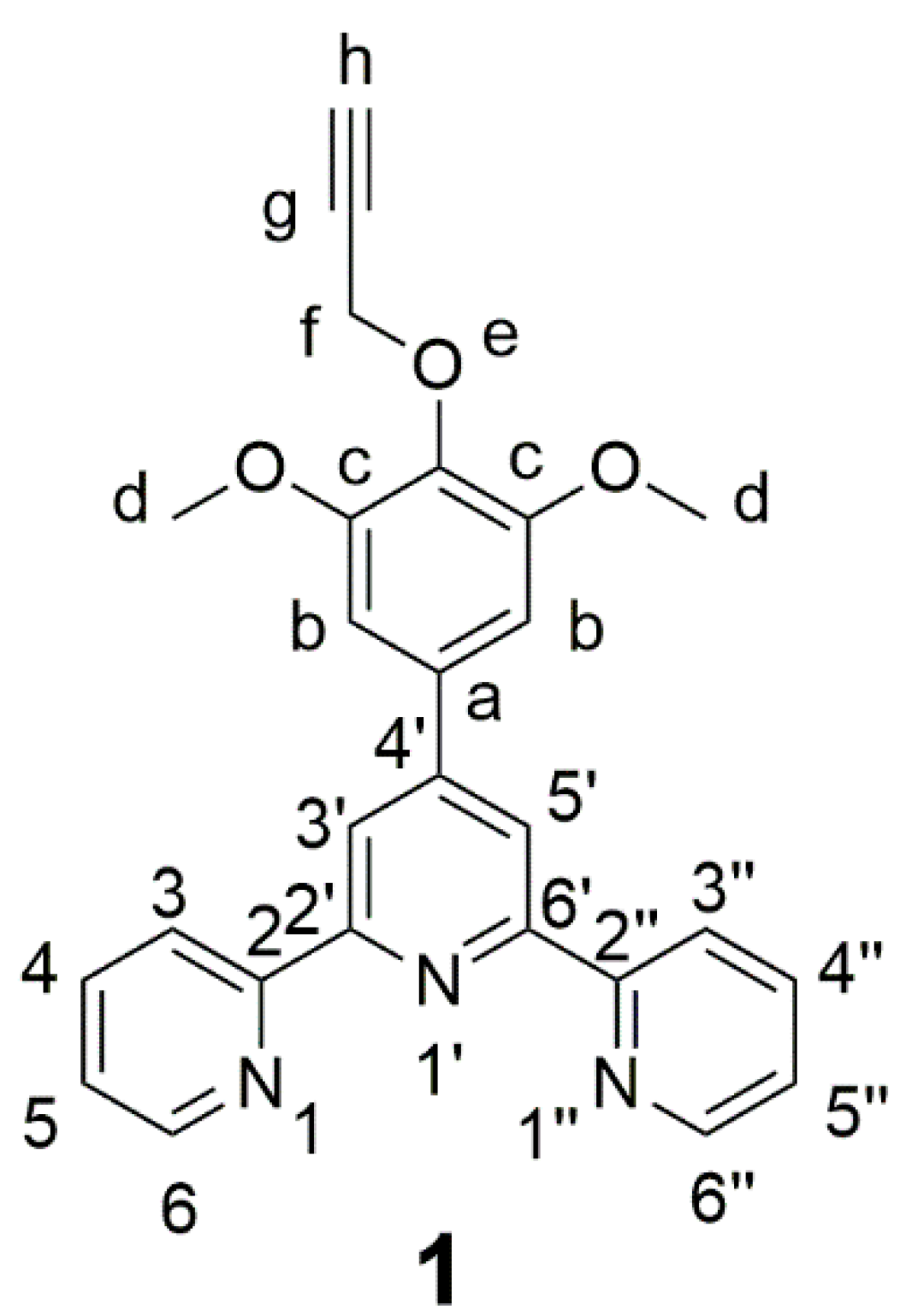
4′-(3,5-Dimethoxy-4-propargyloxyphenyl)-2,2′:6′,2″-terpyridine
Abstract
1. Introduction
2. Results and Discussion
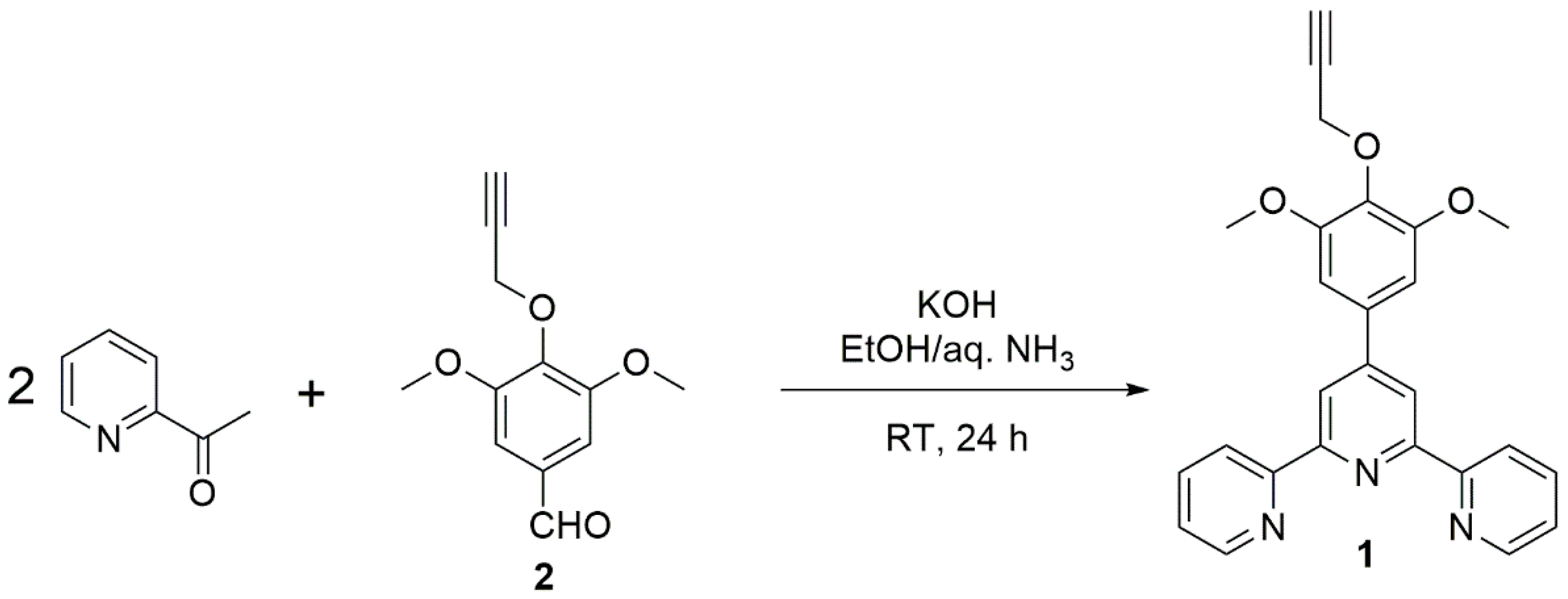
2.1. Synthesis
2.2. Characterization
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Schubert, U.S.; Winter, A.; Newkome, G.R. Terpyridine-Based Materials: For Catalytic, Optoelectronic and Life Sciences Applications; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Vilà, N.; Walcarius, A. Bis(terpyridine) Iron(II) Functionalized Vertically-Oriented Nanostructured Silica Films: Toward Electrochromic Materials. Front. Chem. 2021, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Busemann, A.; Araman, C.; Flaspohler, I.; Pratesi, A.; Zhou, X.-Q.; van Rixel, V.H.S.; Siegler, M.A.; Messori, L.; van Kasteren, S.I.; Bonnet, S. Alkyne Functionalization of a Photoactivated Polypyridyl Complex for Click-Enabled Serum Albumin interaction Studies. Inorg. Chem. 2020, 59, 7710–7720. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, S.; Zhang, Z.; Hao, X.Q.; Jiang, X.; Yu, H.; Wang, P.S.; Xu, B.; Wang, M.; Tian, W.J. Tetraphenylethylene-Based Emissive Supramolecular Metallacages Assembled by Terpyridine Ligands. CCS Chem. 2020, 2, 337–348. [Google Scholar] [CrossRef]
- Voss, F.; Vogt, F.; Herdtweck, E.; Bach, T. Synthesis of Catalytically Active Ruthenium Complexes with a Remote Chiral Lactam as Hydrogen-Bonding Motif. Synthesis 2011, 6, 961–971. [Google Scholar] [CrossRef]
- Davidson, R.J.; Wilson, L.E.; Duckworth, A.R.; Yufit, D.S.; Beeby, A.; Low, P.J. Alkyne substituted mononuclear photocatalysts based on [RuCl(bpy)(tpy)](+). Dalton Trans. 2015, 44, 11368–11379. [Google Scholar] [CrossRef]
- Breul, A.M.; Kübel, J.; Häupler, B.; Friebe, C.; Hager, M.D.; Winter, A.; Dietzek, B.; Schubert, U.S. Synthesis and Characterization of Poly(phenylacetylene)s with Ru(II) Bis-Terpyridine Complexes in the Side-Chain. Macromol. Rapid. Commun. 2014, 35, 747–751. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Li, Y.; Geng, L.; Ren, J.; Feng, G. Fluorescent and Electrochemical Supramolecular Coordination Polymer Hydrogels Formed from Ion-Tuned Self-Assembly of Small Bis-Terpyridine Monomer. Inorg. Chem. 2017, 56, 7512–7518. [Google Scholar] [CrossRef]
- Khatyr, A.; Ziessel, R. Chiral Bipyridine and Terpyridine Ligands Grafted with L-Tyrosine Fragments. Synthesis 2001, 11, 1665–1670. [Google Scholar] [CrossRef]
- Kalachova, L.; Pohl, R.; Hocek, M. Synthesis of nucleoside mono- and triphosphates bearing oligopyridine ligands, their incorporation into DNA and complexation with transition metals. Org. Biomol. Chem. 2012, 10, 49–55. [Google Scholar] [CrossRef]
- Zych, D.; Slodek, A.; Matussek, M.; Filapek, M.; Szafraniec-Gorol, G.; Maslanka, S.; Krompiec, S.; Kotowicz, S.; Schab-Balcerzak, E.; Smolarek, K.; et al. 4′-Phenyl-2,2′:6′,2″-terpyridine derivatives-synthesis, potential application and the influence of acetylene linker on their properties. Dye. Pigment. 2017, 146, 331–343. [Google Scholar] [CrossRef]
- Zhu, B.-H.; Liu, Y.-H.; Jin, X.-Y.; Xu, H.-Y.; Han, Y.-Y.; Zhao, Q. Synthesis, characterization and luminescence properties of Ln(III) (Ln = Eu, Tb, Ce, Sm, Dy) complexes containing a terpyridine ligand and a 3d-4f type conjugated terpyridine-alkyne bridging Eu-III-Co-0 carbonyl cluster complex. Polyhedron 2014, 74, 67–71. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Neuburger, C.E.; Schaffner, S.; Shardlow, E.J. Selective addressing of heteroditopic ligands by iron(II) and platinum(II). Inorg. Chim. Acta 2007, 360, 4069–4076. [Google Scholar] [CrossRef]
- Ziessel, R.; Diring, S.; Retailleau, P. Terpyridine-platinum(II) acetylide complexes bearing pendent coordination units. Dalton Trans. 2006, 27, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Housecroft, C.E.; Johnston, L.A.; Armspach, D.; Neuburger, M.; Zehnder, M. Dicobalt cluster functionalized 2,2′:6′,2″-terpyridine ligands and their ruthenium(II) complexes. Polyhedron 2001, 20, 483–492. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Johnston, L.A. Dicobalt cluster-functionalized 2,2′:6′,2″-terpyridine ligands: Ruthenium(II) complexes with covalently linked C2Co2(CO)6 units. Inorg. Chem. Commun. 1998, 1, 68–70. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Sriprasanthi, R.B.; Shamsudeen, S.; Adam, F.; Bhawani, S.A. A Concise Review of the Natural Existence, Synthesis, Properties, and Applications of Syringaldehyde. Bioresources 2012, 7, 4377–4399. [Google Scholar]
- Dehaudt, J.; Husson, J.; Guyard, L. A more efficient synthesis of 4,4′,4″-tricarboxy-2,2′:6′,2″-terpyridine. Green Chem. 2011, 13, 3337–3340. [Google Scholar] [CrossRef]
- Husson, J.; Dehaudt, J.; Guyard, L. Preparation of carboxylate derivatives of terpyridine via the furan pathway. Nat. Protoc. 2014, 9, 21–26. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. 4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine: A New Ligand Obtained from a Biomass-Derived Aldehyde with Potential Application in Metal-Catalyzed Reactions. Molbank 2018, 2018, 1032. [Google Scholar] [CrossRef]
- Mongal, B.N.; Bhattacharya, S.; Sengupta, S.; Mandal, T.K.; Datta, J.; Naskar, S. A novel ruthenium sensitizer with –OMe substituted phenyl-terpyridine ligand for dye sensitized solar cells. Sol. Energy 2016, 134, 107–118. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.W.C. The synthesis of 2,2′:6′,2″-terpyridine ligands- versatile building blocks for supramolecular chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Heller, M.; Schubert, U.S. Syntheses of functionalized 2,2′:6′,2″-terpyridines. Eur. J. Org. Chem. 2003, 6, 947–961. [Google Scholar] [CrossRef]
- Fallahpour, R.A. Synthesis of 4′-substituted-2,2′:6′,2″-terpyridines. Synthesis 2003, 2, 155–184. [Google Scholar] [CrossRef]
- Kröhnke, F. Synthesis using pyridinium salts.5. Specific synthesis of pyridines and oligopyridines. Synthesis 1976, 1, 1–24. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A Facile Route to Sterically Hindered and Non-Hindered 4′-Aryl-2,2′:6′,2″-Terpyridines. Synlett 2005, 8, 1251–1254. [Google Scholar] [CrossRef]
- Razzano, V.; Paolino, M.; Reale, A.; Giuliani, G.; Artusi, R.; Caselli, G.; Visintin, M.; Makovec, F.; Donati, A.; Villafiorita-Monteleone, F.; et al. Development of Imidazole-Reactive Molecules Leading to a New Aggregation-Induced Emission Fluorophore Based on the Cinnamic Scaffold. ACS Omega 2017, 2, 5453–5459. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. Trac-Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. 4′-(N-(propargyl)pyrrol-2-yl)-2,2′:6′,2″-terpyridine. Molbank 2022, 2022, 1356. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chameroy, R.; Deboskre, C.; Husson, J.; Jourdain, I.; Knorr, M. 4′-(3,5-Dimethoxy-4-propargyloxyphenyl)-2,2′:6′,2″-terpyridine. Molbank 2022, 2022, M1527. https://doi.org/10.3390/M1527
Chameroy R, Deboskre C, Husson J, Jourdain I, Knorr M. 4′-(3,5-Dimethoxy-4-propargyloxyphenyl)-2,2′:6′,2″-terpyridine. Molbank. 2022; 2022(4):M1527. https://doi.org/10.3390/M1527
Chicago/Turabian StyleChameroy, Romain, Clément Deboskre, Jérôme Husson, Isabelle Jourdain, and Michael Knorr. 2022. "4′-(3,5-Dimethoxy-4-propargyloxyphenyl)-2,2′:6′,2″-terpyridine" Molbank 2022, no. 4: M1527. https://doi.org/10.3390/M1527
APA StyleChameroy, R., Deboskre, C., Husson, J., Jourdain, I., & Knorr, M. (2022). 4′-(3,5-Dimethoxy-4-propargyloxyphenyl)-2,2′:6′,2″-terpyridine. Molbank, 2022(4), M1527. https://doi.org/10.3390/M1527






