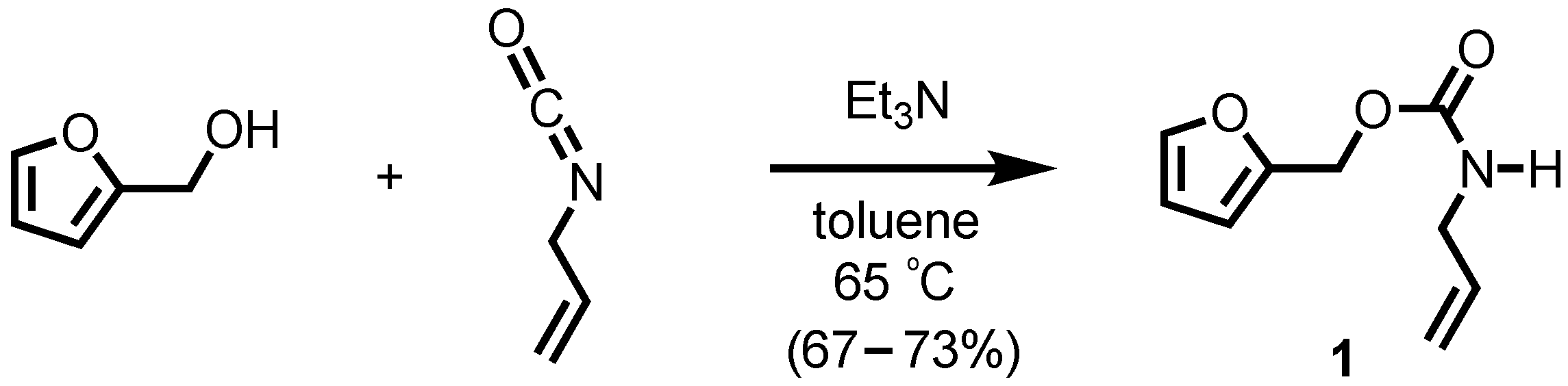
2-Furanylmethyl N-(2-propenyl)carbamate
Abstract
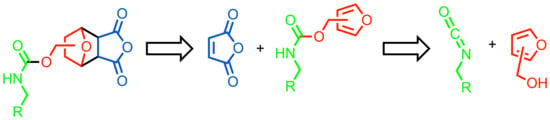
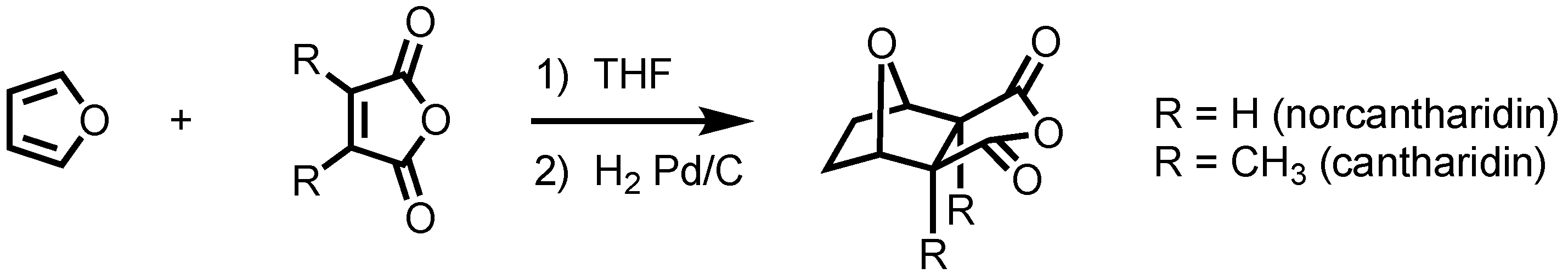
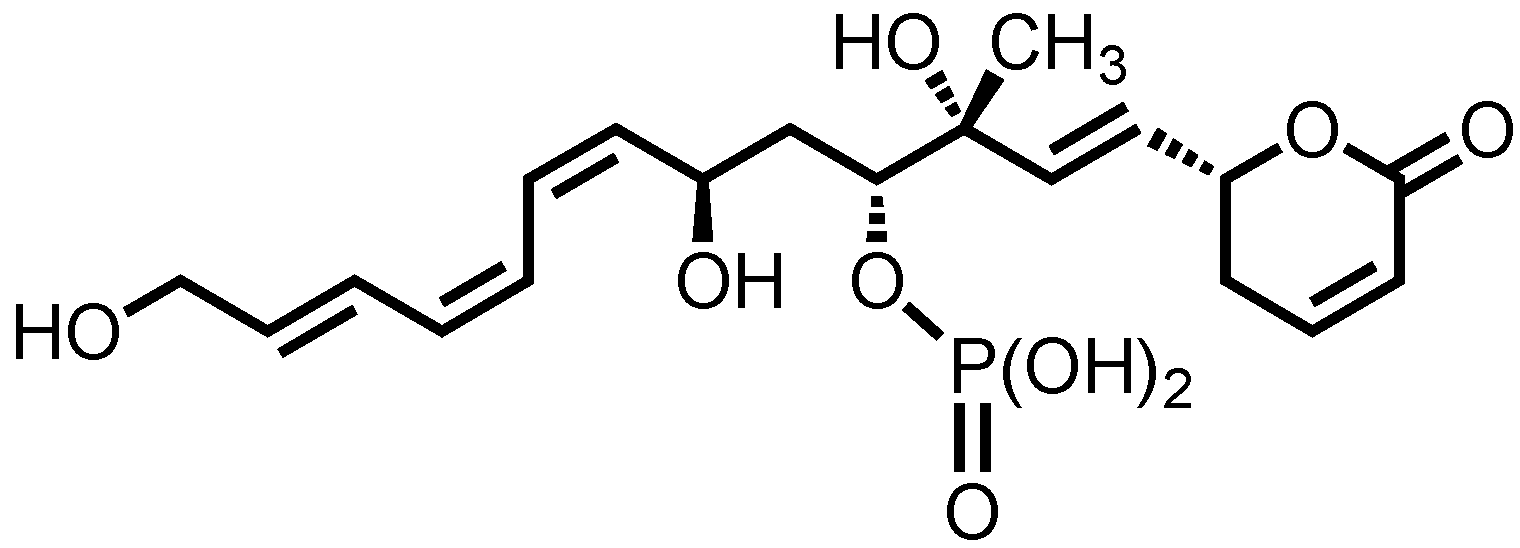
1. Introduction
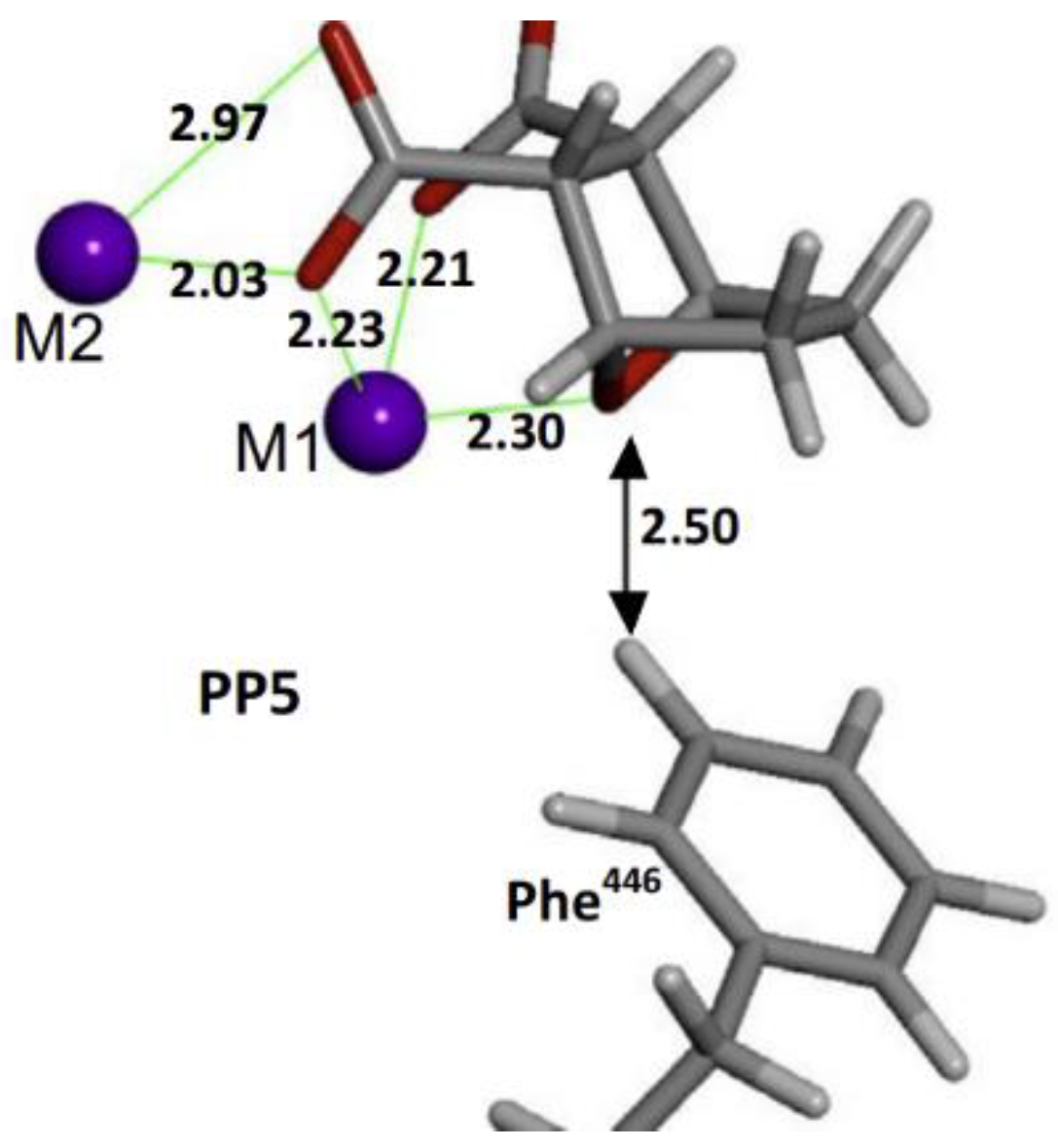
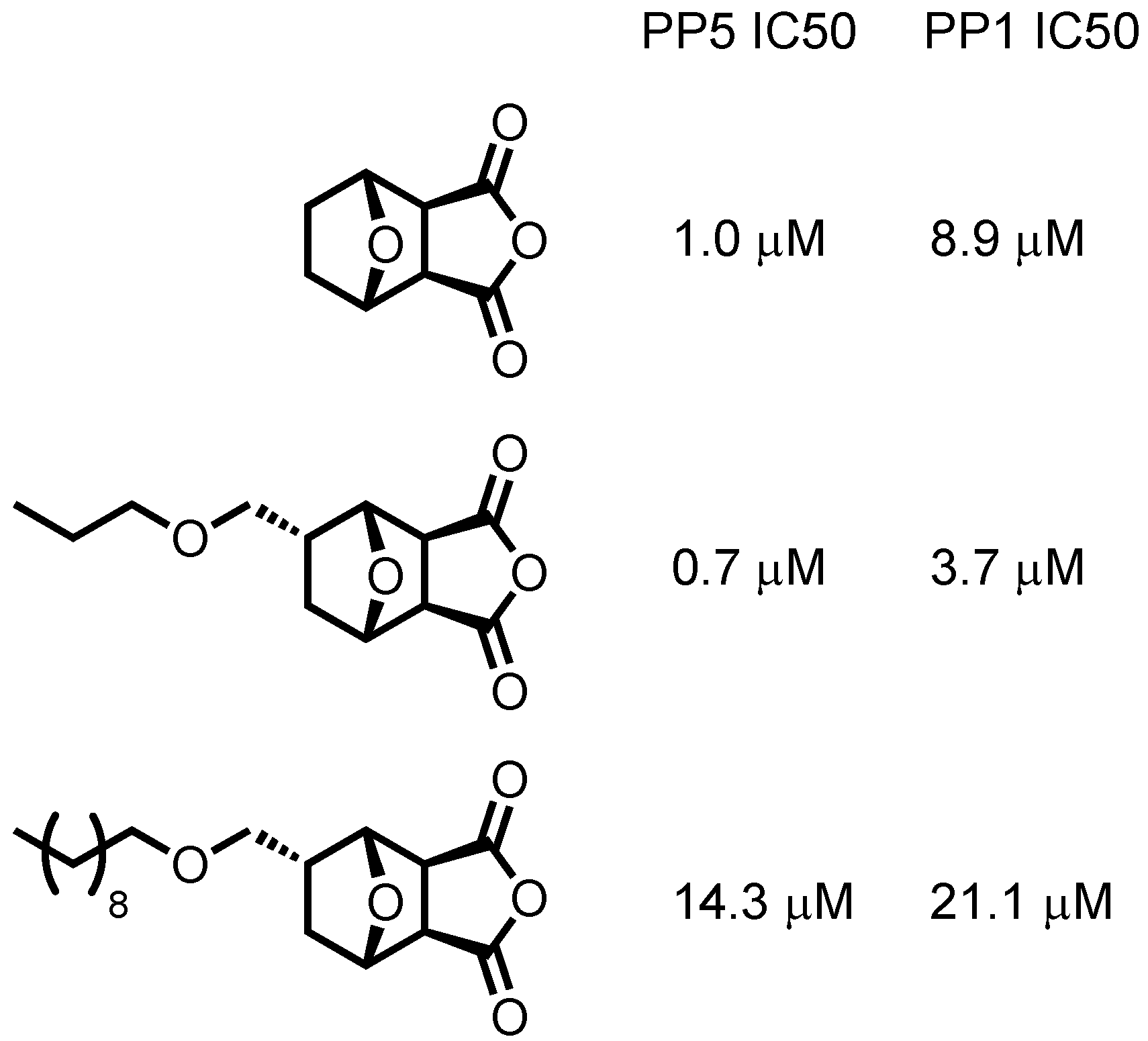
2. Background
3. Results and Discussion
4. Materials and Methods
5. Experimental
2-Furanylmethyl N-(2-propenyl)carbamate (1)
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moorhead, G.B.; Trinkle-Mulcahy, L.; Ulke-Lemee, A. Emerging roles of nuclear protein phosphatases. Nat. Rev. Mol. Cell Biol. 2007, 8, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Golden, T.; Swingle, M.; Honkanen, R.E. The role of serine/threonine protein phosphatase type 5 (PP5) in the regulation of stress-induced signaling networks and cancer. Cancer Metastasis Rev. 2008, 27, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Golden, T.; Aragon, I.V.; Rutland, B.; Tucker, J.A.; Shevde, L.A.; Samant, R.S.; Zhou, G.; Amable, L.; Skarra, D.; Honkanen, R.E. Elevated Levels of Ser/Thr Protein Phosphatase 5 (PP5) in Human Breast Cancer. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2008, 1782, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Silverstein, A.M.; Pratt, W.B.; Chinkers, M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J. Biol. Chem. 1996, 271, 32315–32320. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, T.; Zhu, W.; Dou, L.; Gu, H.; Zhang, L.; Yang, Z.; Chen, H.; Zhou, Q.; Sánchez, E.R.; et al. Protein phosphatase 5 and the tumor suppressor p53 down-regulate each other’s activities in mice. J. Biol. Chem. 2018, 293, 18218–18229. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dong, J.; Deng, L. Overview of Cantharidin and Its Analogues. Curr. Med. Chem. 2018, 25, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.; Swingle, M.R.; Salter, E.A.; Wood, E.; D’Arcy, B.; Zivanov, C.N.; Abney, K.; Musiyenko, A.; Rusin, S.F.; Kettenbach, A.; et al. Crystal Structures and Mutagenesis of PPP-family Ser/Thr Protein Phosphatases Elucidate the Selectivity of Cantharidin and Novel Norcantharidin- Based Inhibitors of PP5C. Biochem. Pharmacol. 2016, 109, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Calderone, V.; Fragai, M.; Luchinat, C.; Talluri, E. Structural Basis of Serine/Threonine Phosphatase Inhibition by the Archetypal Small Molecules Cantharidin and Norcantharidin. J. Med. Chem. 2009, 52, 4838–4843. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Takahashi, N.; Ishi, K.; Kusayanagi, T.; Kuramochi, K.; Sugawara, F. Antitumor antibiotic fostriecin covalently binds to cysteine-269 residue of protein phosphatase 2A catalytic subunit in mammalian cells. Bioorg. Med. Chem. 2009, 17, 8113–8122. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.N.; Sulon, S.M.; Pham, L.N.; Xiang, K.R.; Sykora, R.E.; Forbes, D.C. (1S*,2R*,3S*,4R*,5R*)-5-Tetradecyloxymethyl-7-oxabicyclo [2.2.1]heptane-2,3-dicarboxylicanhydride. Acta Cryst. E 2012, 68, o3374. [Google Scholar] [CrossRef] [PubMed]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, N.C.; Mock, A.L.; Nguyen, I.B.; Patel, S.D.; Forbes, D.C. 2-Furanylmethyl N-(2-propenyl)carbamate. Molbank 2022, 2022, M1510. https://doi.org/10.3390/M1510
Baker NC, Mock AL, Nguyen IB, Patel SD, Forbes DC. 2-Furanylmethyl N-(2-propenyl)carbamate. Molbank. 2022; 2022(4):M1510. https://doi.org/10.3390/M1510
Chicago/Turabian StyleBaker, Noah C., Abby L. Mock, Ivy B. Nguyen, Savan D. Patel, and David C. Forbes. 2022. "2-Furanylmethyl N-(2-propenyl)carbamate" Molbank 2022, no. 4: M1510. https://doi.org/10.3390/M1510
APA StyleBaker, N. C., Mock, A. L., Nguyen, I. B., Patel, S. D., & Forbes, D. C. (2022). 2-Furanylmethyl N-(2-propenyl)carbamate. Molbank, 2022(4), M1510. https://doi.org/10.3390/M1510