N-(p-Toluenesulfonyl)-1-(4′-acetylphenoxy)acrylimidate: Synthesis, Crystal Structure and Theoretical Studies
Abstract
1. Introduction
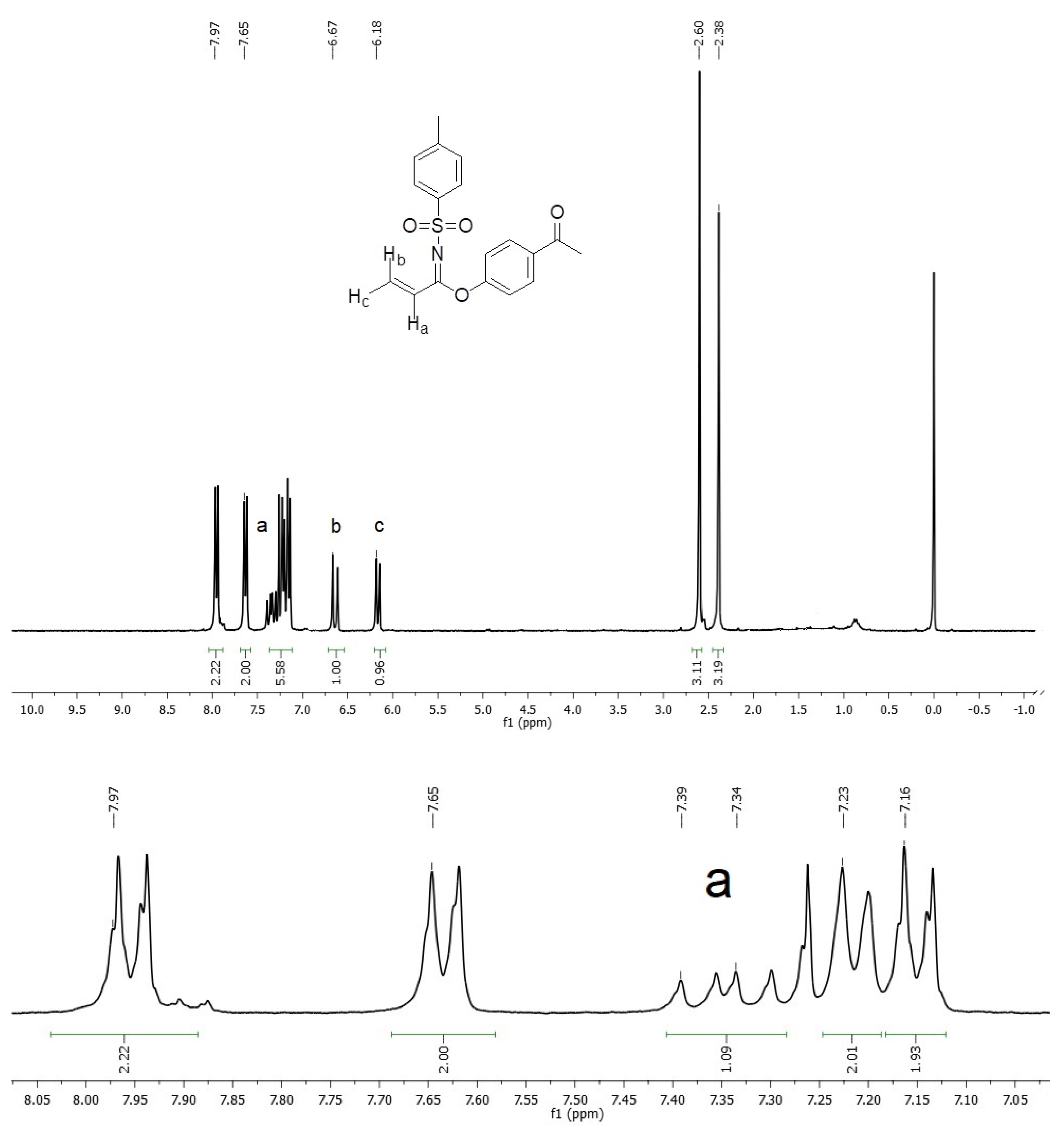
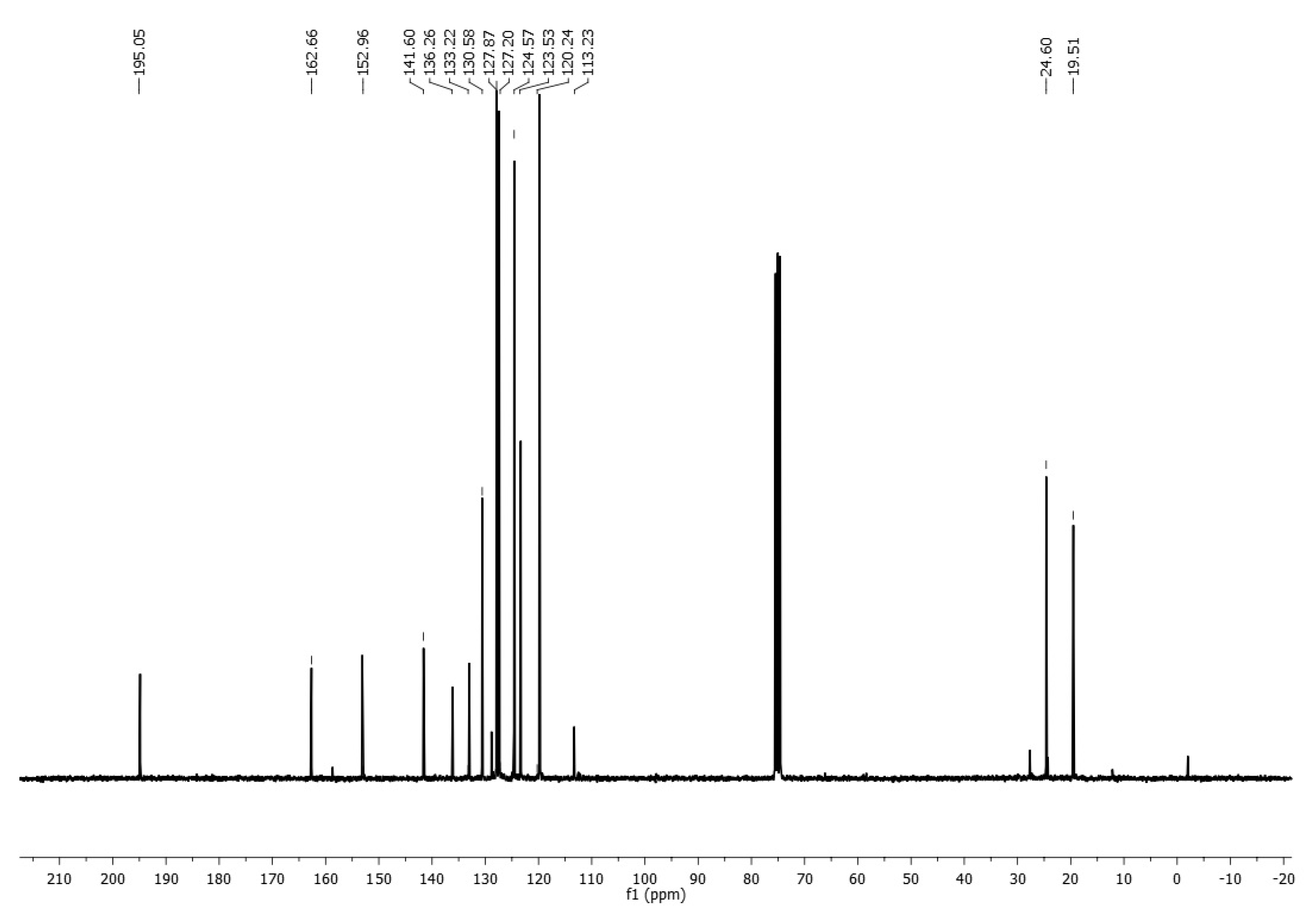
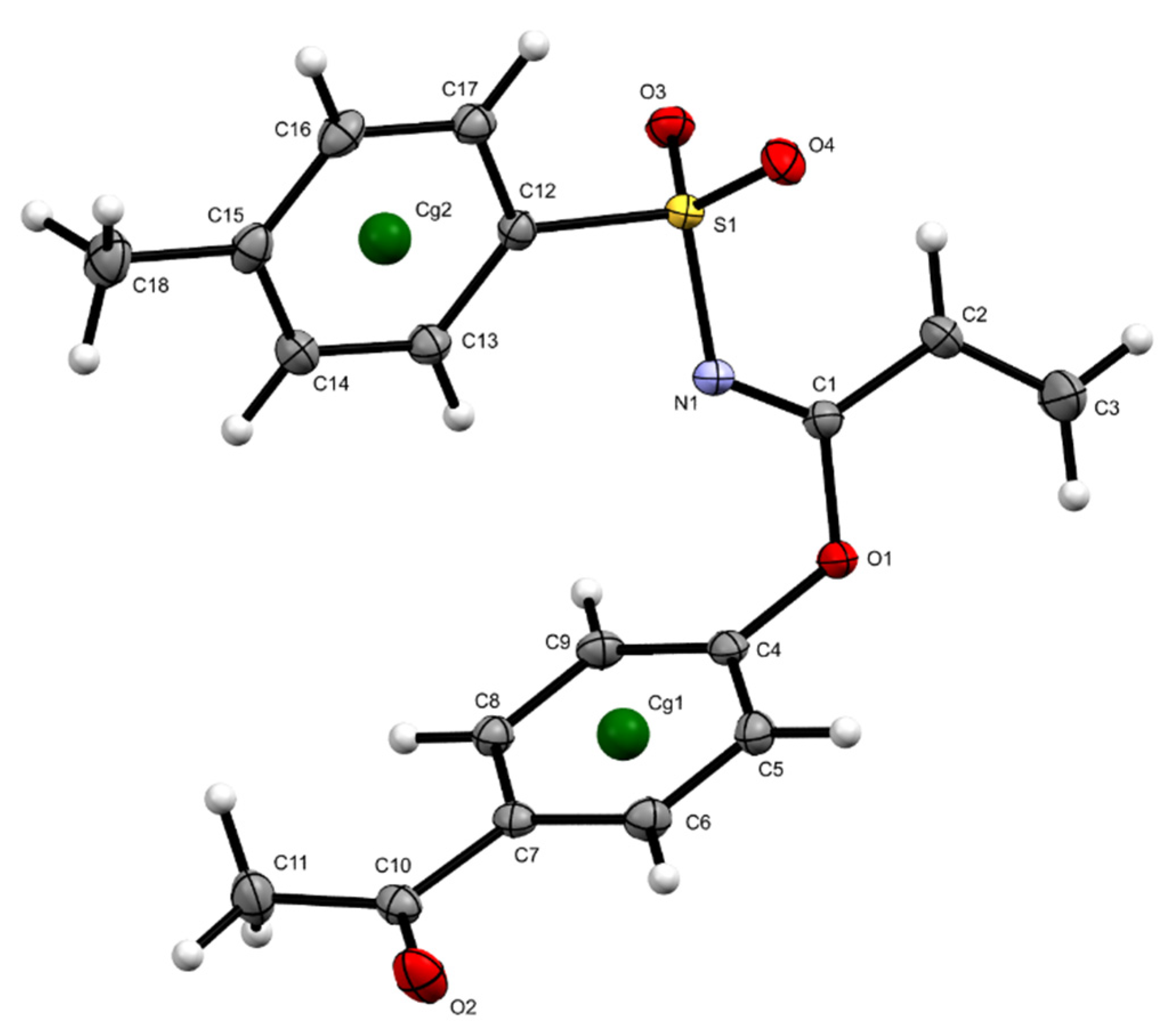
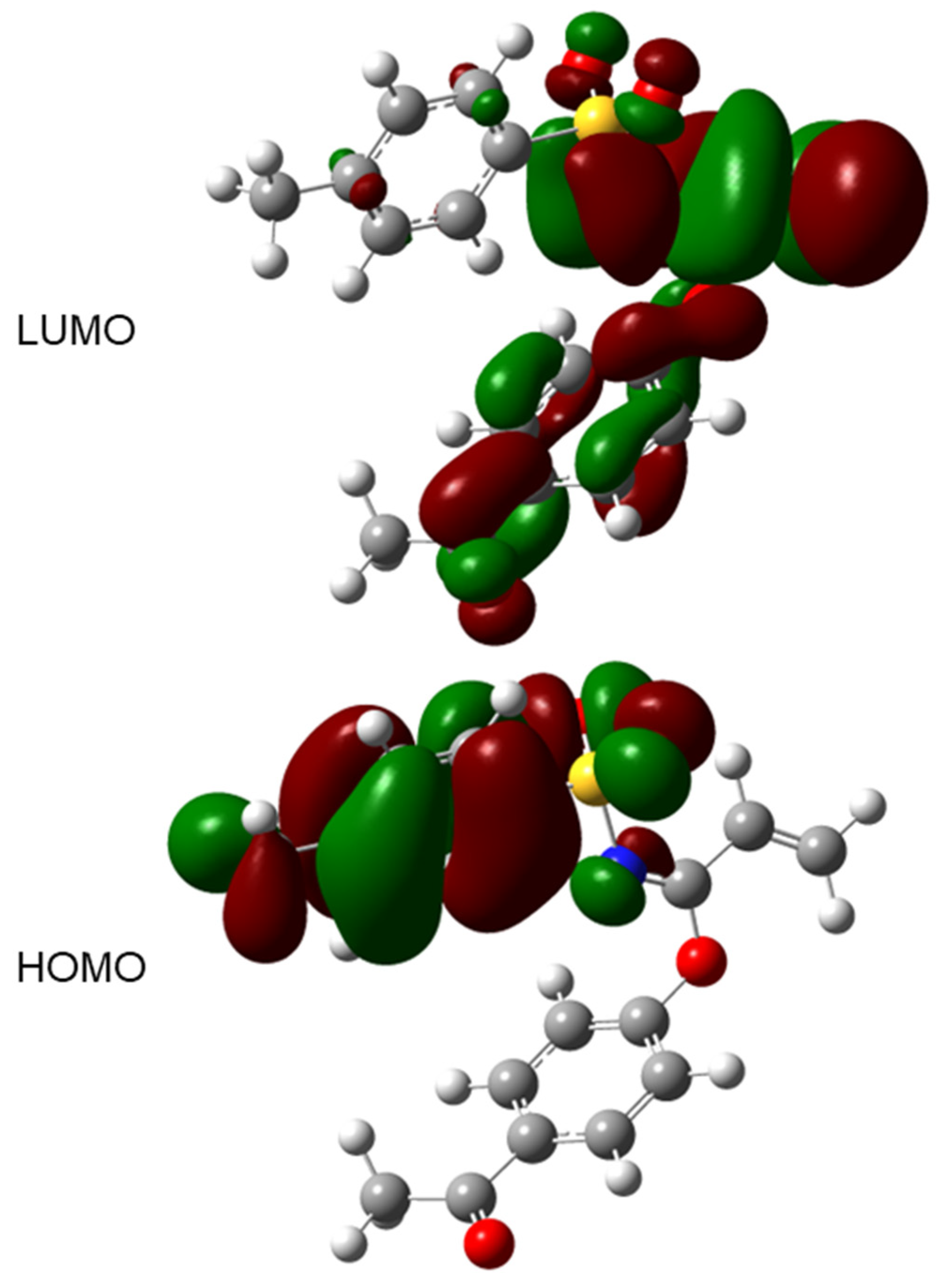
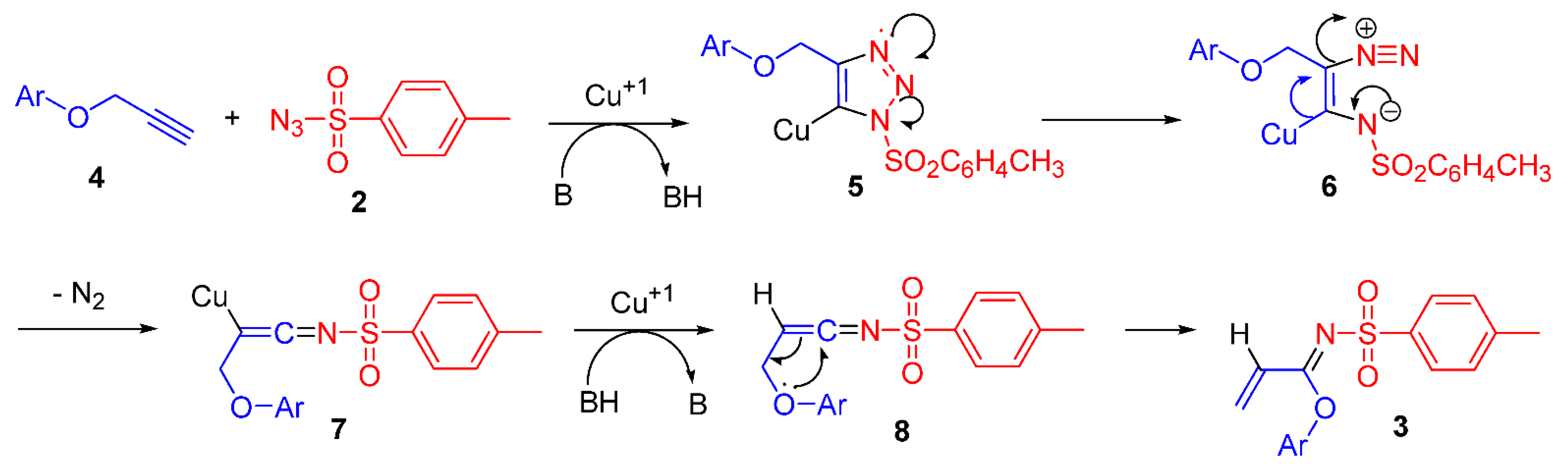
2. Results and Discussion
3. Materials and Methods
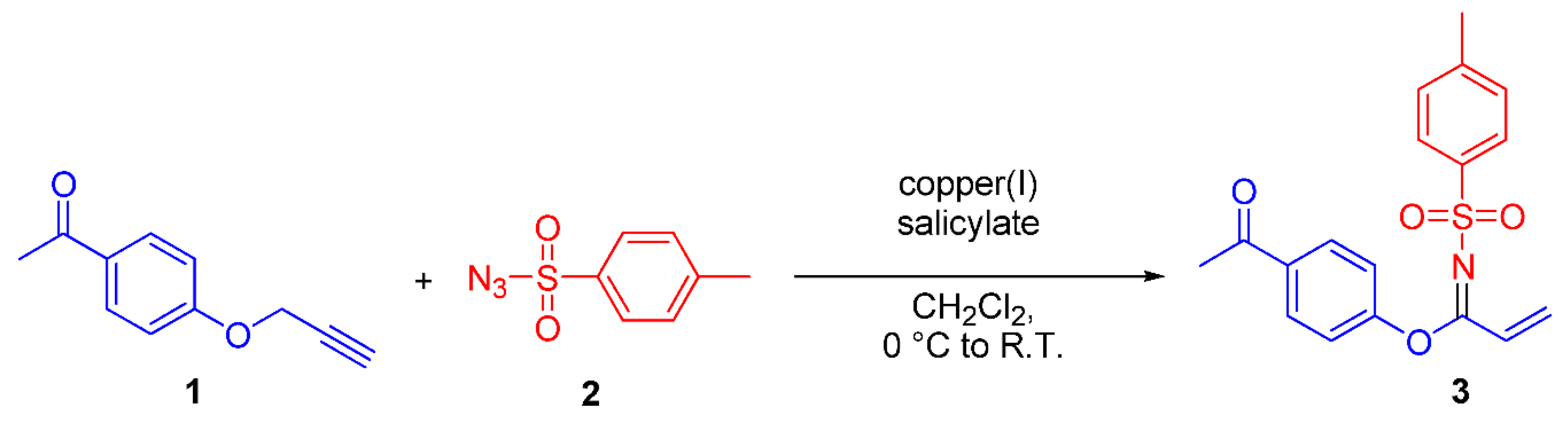
Synthesis of N-(p-Toluenesulfonyl)-1-(4′-acetylphenoxy)acrylimidate 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong Yoo, E.J.; Ahlquist, M.; Bae, I.; Sharpless, K.B.; Fokin, V.V.; Chang, S. Mechanistic Studies on the Cu-Catalyzed Three-Component Reactions of Sulfonyl Azides, 1-Alkynes and Amines, Alcohols, or Water: Dichotomy via a Common Pathway of the article. J. Org. Chem. 2008, 73, 5520–5528. [Google Scholar]
- Kim, S.H.; Park, S.H.; Choi, J.C.; Chang, S. Sulfonyl and Phosphoryl Azides: Going Further Beyond the Click Realm of Alkyl and Aryl Azides. Chem. Asian J. 2011, 6, 2618–2634. [Google Scholar] [CrossRef] [PubMed]
- Valencia, R.A.; Corona, D.; Cuevas-Yañez, E. Effect of the ligand in the Synthesis of 1-Sulfonyl-1,2,3-triazoles through Copper-Catalyzed Alkyne-Azide Cycloaddition. J. Mex. Chem. Soc. 2012, 56, 152–155. [Google Scholar] [CrossRef]
- García-Vanegas, J.J.; Rodríguez-Florencio, J.; Cifuentes-Castañeda, D.D.; Mendieta-Zerón, H.; Pavón-Romero, S.; Morales-Rodríguez, M.; Corona-Becerril, D.; Cuevas-Yañez, E. Synthesis and antifungal activity evaluation of 1-sulfonyl-1,2,3-triazoles. Pharm. Chem. J. 2021, 55, 566–569. [Google Scholar] [CrossRef]
- Cervantes-Reyes, A.; García-Vanegas, J.J.; Lopez-Téllez, G.; Hernández-Balderas, U.; García-Eleno, M.A.; Morales-Morales, D.; Cuevas-Yañez, E. Facile synthesis of copper(I) salicylate and its use in the selective synthesis of 1-acyloxy-1,2,3-triazoles and 1-sulfonyl-1,2,3-triazoles. J. Coord. Chem. 2022, 75, 729–737. [Google Scholar] [CrossRef]
- Hisamatsu, S.; Masu, H.; Azumaya, I.; Takahashi, M.; Kishikawa, K.; Kohmoto, S. U-Shaped Aromatic Ureadicarboxylic Acids as Versatile Building Blocks: Construction of Ladder and Zigzag Networks and Channels. Cryst. Growth Des. 2011, 11, 5387–5395. [Google Scholar] [CrossRef]
- Guo, Y.D.; Yan, X.H.; Xiao, Y.; Liu, C.S. U-shaped relationship between current and pitch in helicene molecules. Sci. Rep. 2015, 5, 16731. [Google Scholar] [CrossRef]
- Kumar, G.R.; Kumar, Y.K.; Kant, R.; Reddy, M.S. Tandem Cu-catalyzed ketenimine formation and intramolecular nucleophile capture: Synthesis of 1,2-dihydro-2-iminoquinolines from 1-(o-acetamidophenyl)propargyl alcohols. Beilstein J. Org. Chem. 2014, 10, 1255–1260. [Google Scholar] [CrossRef]
- Kumar, Y.K.; Kumar, G.R.; Reddy, M.S. Cu-Catalyzed Conversion of Propargyl Acetates to E-α,β-Unsaturated Amides via Ketenimine Formation with Sulfonyl Azides. J. Org. Chem. 2014, 79, 823–828. [Google Scholar] [CrossRef]
- Chauhan, D.P.; Varma, S.J.; Vijeta, A.; Banerjee, P.; Talukdar, P. A 1,3-amino group migration route to form acrylamidines. Chem. Commun. 2014, 50, 323–325. [Google Scholar] [CrossRef]
- Bruker. APEX2, SAINT, & SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; Mccabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Cason, C.; Fröhlich, T.; Lipka, C. Persistence of Vision Raytracer. 2013. Available online: http://www.povray.org/ (accessed on 1 January 2020).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01, Wallingford, CT. 2016. Available online: https://www.scirp.org/(S(lz5mqp453ed%20snp55rrgjct55))/reference/referencespapers.aspx?referenceid=2418053 (accessed on 1 January 2020).
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar]
- Spackman, P.R.; Turner, M.J.; Mckinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graphics 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Kelley, C.; Bersch, C.; Bröker, H.-B.; Campbell, J.; Cunningham, R.; Denholm, D.; Elber, G.; Fearick, R.; Grammes, C.; et al. gnuplot 5.2: An Interactive Plotting Program. 2019. Available online: http://www.gnuplot.info (accessed on 1 January 2020).

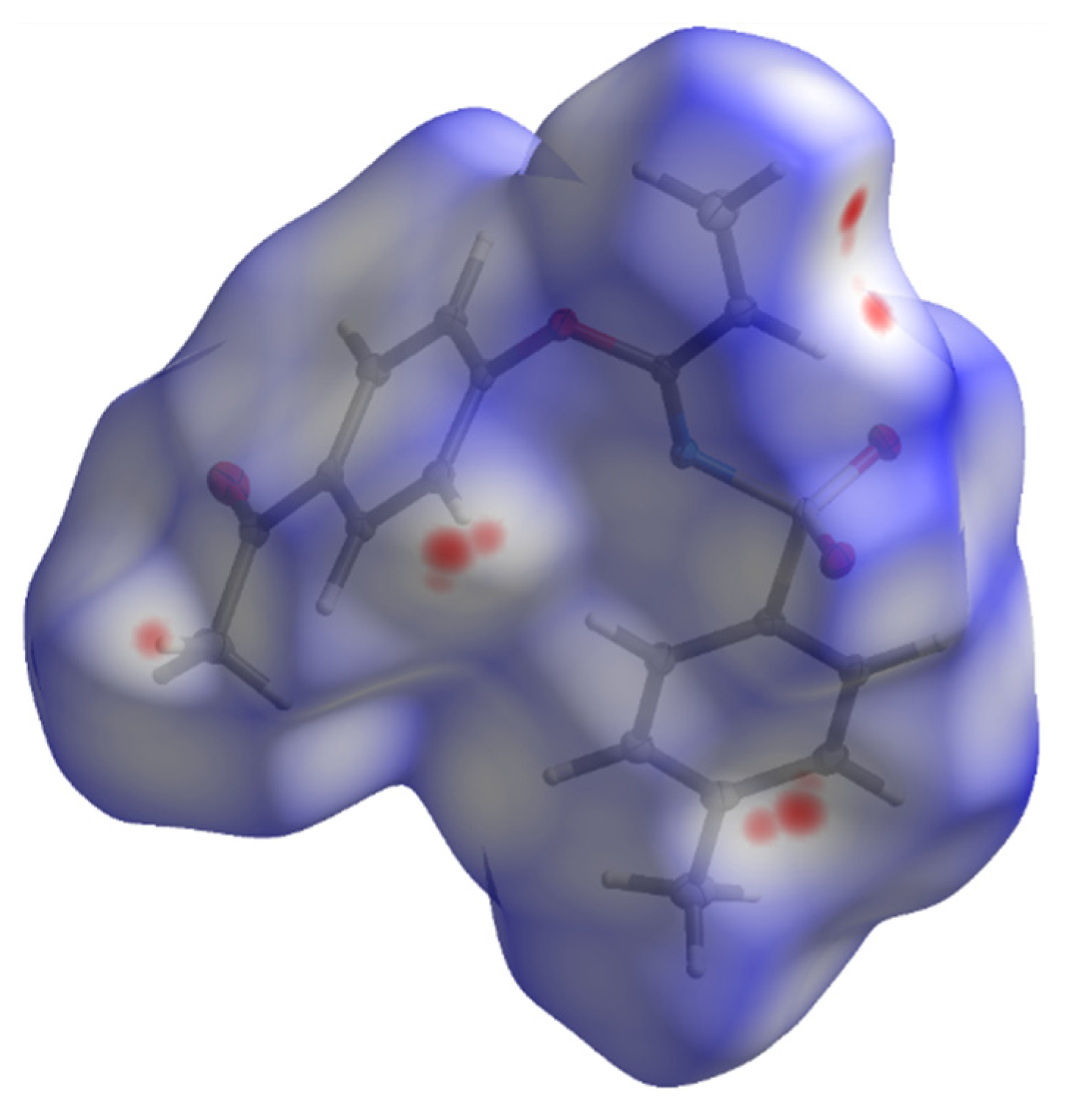


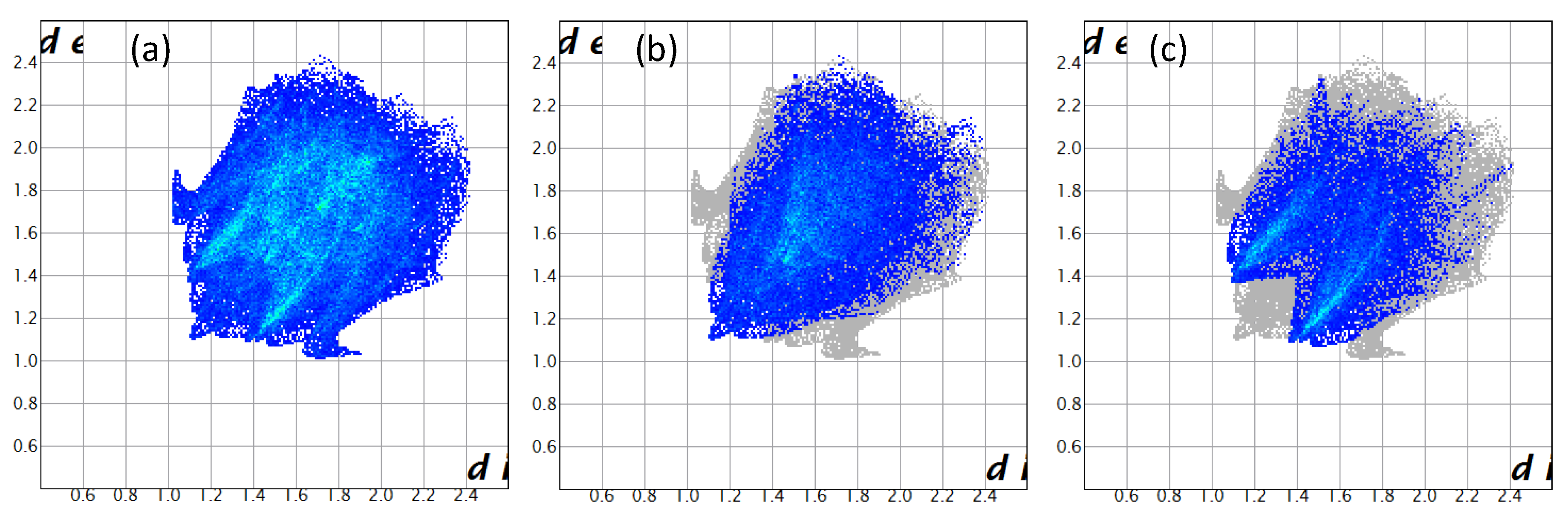
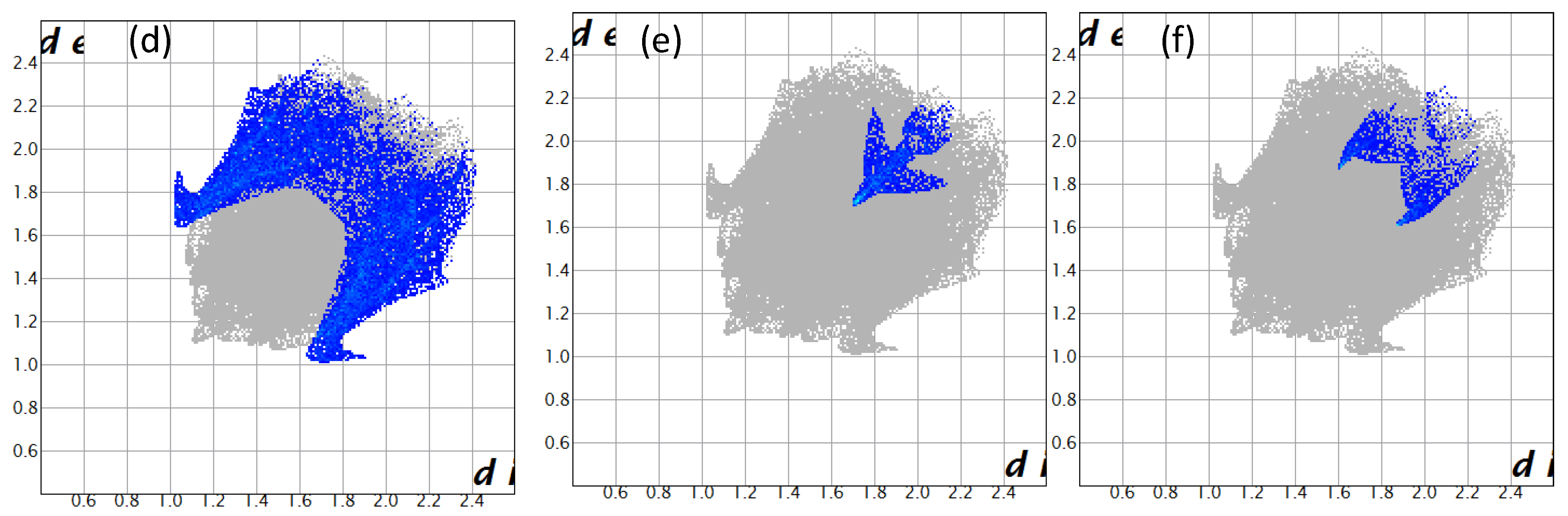
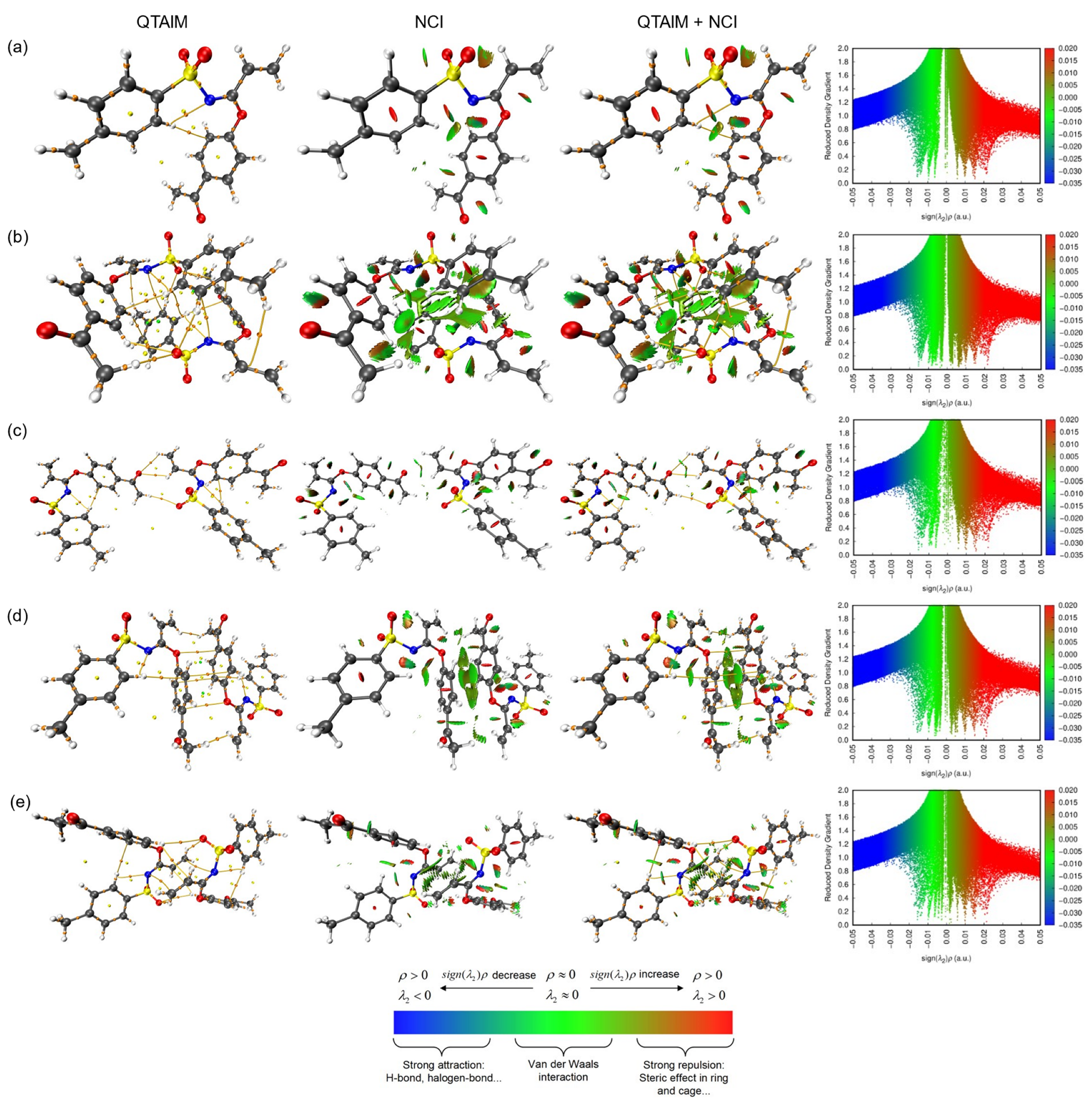


| Crystal Data | 3 |
|---|---|
| Empirical formula | C18H17NO4S |
| Formula weight | 343.38 |
| Temperature (K) | 100(2) |
| Radiation type | Mo Kα |
| Crystal system | Triclinic |
| Space group | P − 1 |
| Unit cell dimensions (Å, °) | |
| a | 9.1339(4) |
| b | 9.4474(4) |
| c | 10.7404(4) |
| α | 66.3790(10) |
| β | 85.4950(10) |
| γ | 77.8620(10) |
| Volume (Å3) | 830.15(6) |
| Z | 2 |
| Density (calculated, Mg/m3) | 1.374 |
| Absorption coefficient μ (mm−1) | 0.217 |
| F(000) | 360 |
| Crystal size (mm3) | 0.160 × 0.185 × 0.318 |
| Θ range (deg) | 2.070 to 27.504 |
| Index ranges | −11 ≤ h ≤ 11, −12 ≤ k ≤ 12, −13 ≤ l ≤ 13 |
| Reflections collected | 18,221 |
| Independent reflections | 3808 [R(int) = 0.0130] |
| Data/restraints/parameters | 3808/0/219 |
| Goodness-of-fit on F2 | 1.039 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0298, wR2 = 0.0805 |
| R indices (all data) | R1 = 0.0306, wR2 = 0.0812 |
| Largest diff. peak and hole (e Å−3) | 0.435, −0.390 |
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| C2—H2···O2 i | 0.95 | 2.59 | 3.2198(14) | 123.9 |
| C3—H3B···O2 i | 0.95 | 2.56 | 3.1986(16) | 124.7 |
| C2—H2···O3 | 0.95 | 2.71 | 3.2620(14) | 117.3 |
| C13—H13···N1 | 0.95 | 2.45 | 2.8398(14) | 104.4 |
| C11—H11A···O4 ii | 0.98 | 2.63 | 3.5461(16) | 155.5 |
| C11—H11B···O3 iii | 0.98 | 2.85 | 3.6760(15) | 142.8 |
| C17—H17···O3 iv | 0.95 | 2.78 | 3.7080(14) | 164.4 |
| C9—H9···Cg2 iii | 0.95 | 2.55 | 3.4644(12) | 162.9 |
| Crystal Data | 3 |
|---|---|
| Etotal (a.u.) | −1448.480 |
| ELUMO (eV) | −1.956 |
| EHOMO (eV) | −7.106 |
| Electron affinity (eV) | 1.956 |
| Ionization potential (eV) | 7.106 |
| Gap Energy (eV) | 5.150 |
| Electronegativity (eV) | 4.531 |
| Chemical hardness (eV) | 2.575 |
| Chemical softness (eV−1) | 0.388 |
| Chemical potential (eV) | −4.531 |
| Electrophilicity index (eV) | 3.986 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escandón-Mancilla, F.M.; Cedillo-Cruz, A.; Gordillo-Cruz, R.E.; Martínez-Otero, D.; Unnamatla, M.V.B.; Cuevas-Yañez, E. N-(p-Toluenesulfonyl)-1-(4′-acetylphenoxy)acrylimidate: Synthesis, Crystal Structure and Theoretical Studies. Molbank 2022, 2022, M1509. https://doi.org/10.3390/M1509
Escandón-Mancilla FM, Cedillo-Cruz A, Gordillo-Cruz RE, Martínez-Otero D, Unnamatla MVB, Cuevas-Yañez E. N-(p-Toluenesulfonyl)-1-(4′-acetylphenoxy)acrylimidate: Synthesis, Crystal Structure and Theoretical Studies. Molbank. 2022; 2022(4):M1509. https://doi.org/10.3390/M1509
Chicago/Turabian StyleEscandón-Mancilla, Flor María, Alberto Cedillo-Cruz, Raúl Eduardo Gordillo-Cruz, Diego Martínez-Otero, M. V. Basavanag Unnamatla, and Erick Cuevas-Yañez. 2022. "N-(p-Toluenesulfonyl)-1-(4′-acetylphenoxy)acrylimidate: Synthesis, Crystal Structure and Theoretical Studies" Molbank 2022, no. 4: M1509. https://doi.org/10.3390/M1509
APA StyleEscandón-Mancilla, F. M., Cedillo-Cruz, A., Gordillo-Cruz, R. E., Martínez-Otero, D., Unnamatla, M. V. B., & Cuevas-Yañez, E. (2022). N-(p-Toluenesulfonyl)-1-(4′-acetylphenoxy)acrylimidate: Synthesis, Crystal Structure and Theoretical Studies. Molbank, 2022(4), M1509. https://doi.org/10.3390/M1509







